This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Oncology drugs estimated to be $1 billion cheaper to develop through a precision approach
Research and development (R&D) costs for targeted oncology drugs are markedly lower if guided by a companion diagnostic strategy, but current biopharmaceutical business models suggest these savings will not be reflected in more affordable pricing.
A new analysis exploring the finances of bringing new cancer drugs to market has found that precision oncology drugs could be $1 billion cheaper to develop than non-precision drugs.
Despite this, there is no evidence that these savings will flow into lower downstream prices for more affordable cancer medicines due to the commercial strategies of pharmaceutical companies.
The paper, published recently in the Journal of Pharmaceutical Policy and Practice, shares the results of a three-year program between Institute of Cancer Policy and Queen's University Belfast that represents the most comprehensive analyzes of the R&D costs of oncology medicines to date.
Precision medicines are drugs that are developed with a companion diagnostic (CDx). A CDx in oncology is a type of medical test that analyzes specific genetic and molecular characteristics of a tumor, what drives the tumor, a patient's biomarkers that indicate they would be a good match for treatment, and more.
Previous analyses have indicated that oncology is the most expensive R&D medicines domain, and companies' strategies are based on driving premium pricing and commercial approaches that threaten to undermine health care budgets. Data from the study demonstrates that it costs over $1 billion more in R&D spend to develop an oncology medicine that is not guided through clinical trials with a CDx, compared to a precision approach.
Precision oncology represents enormous potential R&D savings, but the complexity of the global pharmaceutical industry and companies' commercial strategies mean downstream costs are unlikely to decrease without change. The authors call for companies to adhere to transparent accounting standards for their R&D budgets so health systems can make well-informed policy decisions.
In carrying out the study, the authors looked at the cost of R&D and the return of investment (RoI) of cancer drugs. To do this, they obtained data from the US Securities and Exchange Commission (SEC) on all FDA cancer medicines launched between 1997 and 2020. This included a range of targeted therapies and immuno-oncology drugs that fight against the tumor by instigating immune system action.
The paper also found enormous variation in the R&D costs and returns on investment (RoI) on oncology drugs. The mean cost of developing a cancer medicine was 4.4 billion. However, this hides a huge range of costs. For example, Dinutuximab, a drug used for post-consolidation therapy for childhood risk neuroblastoma, cost just $276 million to develop. On the other hand, the drug Durvalumab cost $13.4 billion, while Isatuximab, cost $15.8 billion.
The researchers also revealed how the mere act of bringing any cancer medicine to market generates substantial returns to a company, regardless of its RoI.
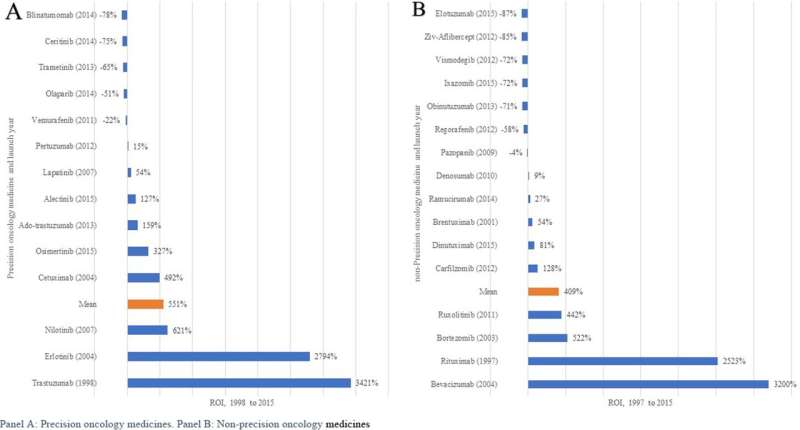
Overall, the RoI for cancer medicines launched between 1997 and 2015 is between 435 and 551%. Similarly to R&D costs, this hides huge variation. A substantial number of cancer medicines to date have negative or flat ROIs as low as minus 78-87%.
However, some cancer medicines launched in the late 1990s to mid-2000s have generated astronomical RoIs. These include pancreatic and lung cancer drug Erlotinib (2,794%), breast cancer drug Trastuzumab (3,421%), Non-Hodgkins Lymphoma drug Rituximab (2,523%), and Bevacizumab (3,200%), which is used for colon, lung, glioblastoma and renal cell cancers.
Overall the study represents one of the most detailed analysis of cancer biopharmaceutical R&D costs and returns on investment which will, the authors hope, inform future national and transnational policy.
"This study illuminates extraordinary differences in R&D costs per cancer drug, as well as some staggering returns on investment. One thing that is abundantly clear is that there is no connection between the prices set for individual drugs and their prior R&D costs, or subsequent returns on investment," says Professor Richard Sullivan, Director of the Institute of Cancer Policy and Co-Director of the Centre for Conflict & Health Research.
More information: Raymond H. Henderson et al, Delivering the precision oncology paradigm: reduced R&D costs and greater return on investment through a companion diagnostic informed precision oncology medicines approach, Journal of Pharmaceutical Policy and Practice (2023). DOI: 10.1186/s40545-023-00590-9



















