This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Rethinking and rigor brings promising protein for cancer therapy
The elucidation of the role of a critical protein in cell biology is paving the way for alternative cancer treatments. By refuting recent challenges that threatened to invalidate their work, a team of KAUST researchers investigating the protein has cleared the way for the development of targeted drugs.
Targeted therapy is a vital form of cancer treatment; identifying the druggable disease-relevant protein targets will enable us to successfully develop more targeted drugs, says Ph.D. student Samah Al-Harthi.
"For any cancer, there can be a big bad guy," explains Ph.D. student Kacper Szczepski. "Our job is to find out if there is a bad guy, exactly what it does and how to get it."
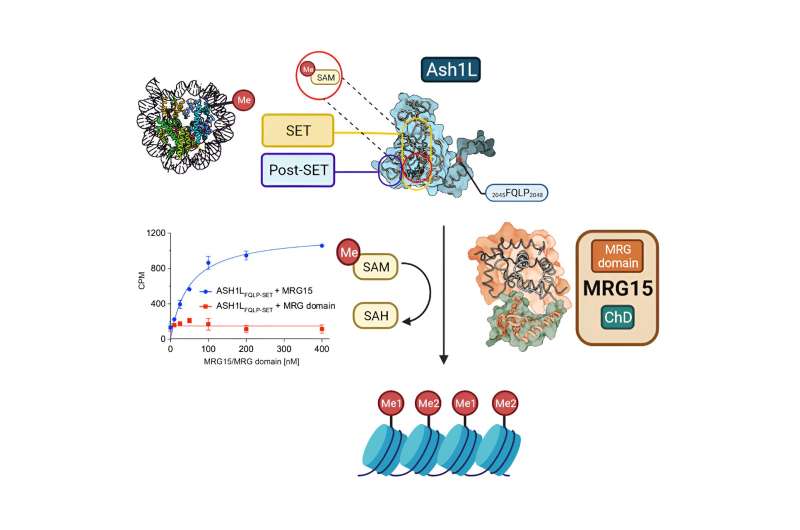
For breast cancer and leukemia, the KAUST team has been studying an oncogene, a protein called ASH1L, since 2018. This protein regulates the genes that control cell division and proliferation, and its overexpression is known to be a key cause of the two cancers.
The team was developing "ligands" for ASH1L, meaning smaller molecules that, as targeted drugs, will selectively attach themselves to the protein and inhibit its production. But then a trio of papers were published by other research groups, and these threatened to derail their approach.
These papers investigated a second protein that commonly associates with ASH1L called MRG15. The papers claimed MRG15 is an activator of ASH1L that also acts to block ASH1L inhibitors. Unlike ASH1L, whose sole function is to regulate genes, MRG15 is known to play at least six other roles in human cells. Targeting it to indirectly affect ASH1L would therefore also interfere with these roles, resulting in side effects for a patient that would rule out such an approach.
"Taken together, these studies gave us more insights into Ash1L enzymatic regulation by an additional co-regulator; however, it also allowed us to carefully consider other players when designing targeted drugs," explains Al-Harthi
As described in a new paper of their own, however, the KAUST team recently conducted rigorous tests with the two proteins. The first author of the paper, Al-Harthi explains that the tests showed that MRG15 is neither an activator of ASH1L, nor does it stop ligands binding to it. "This has affirmed the validity of our earlier work," Al-Harthi said.
"Normally in biology, what you find is that systems are more complex than originally thought. In this case, the system is very simple and there was no need to make it more complex," says team leader Lukasz Jaremko.
Jaremko's team is now researching ASH1L ligands they have found. Once this work has completed its preclinical stage, the researchers hope to receive support from Saudi Arabia's Smart Health Initiative, which will enable them to collaborate with hospitals in the kingdom to develop commercial drugs.
The paper is published in the journal Structure.
More information: Samah Al-Harthi et al, MRG15 activates histone methyltransferase activity of ASH1L by recruiting it to the nucleosomes, Structure (2023). DOI: 10.1016/j.str.2023.07.001