August 30, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists reverse Alzheimer's plaque formation in animal models by boosting activity of key ion channel
Losing the activity of a key ion channel in the brain may contribute to the buildup of a devastating and toxic protein responsible for the clumps of plaque that accumulate in Alzheimer's disease, a team of neurobiologists in China has found.
Stunningly, this team has also shown—at least in animal-model studies—that this protein, a key hallmark of Alzheimer's, can be diminished in the living brain by manipulating the ion channel.
The suspect protein is amyloid-beta, which becomes pervasive in the brain tissue of patients with Alzheimer's disease. Toxic, gooey amyloid-β accumulates in wads between neurons and disrupts the function of these vital brain cells. The ion channel is known simply as TRPM7, and it may contribute to the buildup of toxic amyloid-β when the channel itself ceases to function properly, according to scientists at State Key Laboratory of Medical Neurobiology, Fudan University in Shanghai.
"Toxic aggregation of amyloid-β in neurons is implicated in Alzheimer's disease pathology," asserted lead author Shimeng Zhang, reporting in the journal Science Signaling. Zhang is part of a large team conducting ongoing research into the activity of the TRPM7 ion channel at the lab in Shanghai.
The TRPM7 channel studied by Zhang and colleagues is a special pore in the membrane of neurons, which allows the passage of charged ions from the outer cellular environment into the inner sanctum of the cell. The TRPM7 channel is involved in the uptake of divalent cations, such as Zn2+, Mg2+ and Ca2+ . The ion channel, therefore, helps shape critical cellular activities, such as excitability, plasticity and metabolism.
But TRPM7 is a dual-function protein that is both an ion channel and a kinase. As difficult as it may be to imagine, this complex constituent in the neuronal membrane is also an enzyme—a kinase—that catalyzes the transfer of high-energy phosphate groups from potent ATP molecules to fuel activities involving TRPM7. Malfunctioning TRPM7 ion channels have been linked not just to Alzheimer's disease but various other neurodegenerative diseases, studies have shown.
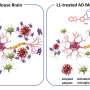
In a portion of the team's study that examined human brain tissue, Zhang and colleagues found that the amount of TRPM7 was substantially low in postmortem brain samples from patients with Alzheimer's disease and in two different mouse models of amyloid-β-induced pathology.
Zhang and colleagues confirmed in their research that losing the activity of ion channel TRPM7 may contribute to the buildup of toxic amyloid proteins in Alzheimer's disease. Their findings may also provide a mechanistic link between the loss of TRPM7 and amyloid pathology in patients afflicted with the mind-robbing condition.
The Shanghai scientists not only revealed in their research that TRPM7 declines in Alzheimer's disease, the team also conducted a series of experiments with mouse models and showed that diminished amounts of TRPM7 in cell membranes resulted in the accumulation of amyloid-β.
Keenly aware that TRPM7 is a dual-functioning protein (an ion channel and a kinase), the team then asked a deceptively simple question: What if we boost the amount of full length TRPM7—ion channel plus kinase—in one group of animals and provide the other group with an abundance of the kinase portion of the molecule?
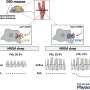
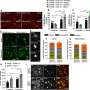
In a group of aged mice that were bred for amyloid-β presence in their brain tissue, the scientists increased the amount of full length TRPM7 in these animals, overexpressing it substantially. In that experiment, the collaborative team found that by boosting the amount of the full length TRPM7 molecules, they were able to restore synapse formation and cognitive function in the mice. The kinase portion of the molecule is known as M7CK.
Then, in younger animals the kinase portion alone was introduced and overexpressed. And when M7CK was tested in this cohort, the kinase directly activated a cascade of activities. For example, the protease, MMP14, promoted amyloid-β degradation and clearance. The kinase actually helped sweep destructive amyloid-β out of the brains of these animals. Indeed, the team—at least in the lab—had found a way to restore synaptic activity and cognitive function in one group of mice and the elimination of toxic amyloid-β in the other.
"We found that the kinase activity of TRPM7 is important to stimulate the degradation of amyloid-β," Zhang reported. "TRPM7 expression was decreased in hippocampal tissue samples from patients with Alzheimer's disease and two mouse models of AD. In cultures of hippocampal neurons from mice, overexpression of full-length TRPM7 or of its functional kinase domain M7CK prevented synapse loss induced by exogenous Aβ."
Overexpression of M7CK maintained cognitive function in presymptomatic young mice and restored synapse formation and cognitive function in aged mice. Further studies revealed that M7CK directly activated the enzyme MMP14, which promoted the degradation and clearance of amyloid-β.
As promising as the new research seems, scientists worldwide have yet to definitively say whether amyloid plaques are a cause of the disease. Although clearly toxic to the brain, the plaque formations may occur in the aftermath of a subtler series of deleterious events that have yet to be discovered.
Additionally, another protein, known simply as tau, forms insoluble stringy filaments in the brain that accumulate as neurofibrillary tangles. Just as some scientists have proposed that plaques are a possible cause of the disease, others have argued in favor of tau.
Ultimately, their synergism may also tell a deeper story about Alzheimer's genesis. Together the amyloid plaques and tau's neurofibrillary tangles create the hallmark plaques and tangles of the disease.
Alzheimer's also has been linked to a number of different gene variants, but most cases occur "sporadically," which means the disorder cannot be traced to a specific cause. And whether genetic or sporadic, sticky amyloid plaques are a defining characteristic of the disorder for which there is no cure. In the U.S. alone, total health care costs for the disorder run $355 billion annually, data from the Alzheimer's Association show.
Unless a cure is found, the disorder is expected to overwhelm global health care systems by 2050 because of the inexorable aging of populations, according to the World Health Organization.
"Our results show that [Alzheimer's disease] pathology is associated with a reduction in TRPM7 expression and that maintaining normal expression of its kinase domain is sufficient to reduce amyloid-β accumulation, protect synaptic density and prevent or reverse memory deficits," Zhang concluded.
More information: Shimeng Zhang et al, TRPM7 kinase activity induces amyloid-β degradation to reverse synaptic and cognitive deficits in mouse models of Alzheimer's disease, Science Signaling (2023). DOI: 10.1126/scisignal.ade6325
© 2023 Science X Network