This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
3D genome analysis reveals secrets to antibody diversity
One of the remaining mysteries of immunology is the exact mechanism that B cells use to generate millions of antibodies with different specificities to protect us from the plethora of pathogens in the environment.
Utilizing research expertise in antibody variation and 3D genome organization, researchers at the Babraham Institute have shown how unique DNA associations in each B cell lie at the heart of antibody variation. With further research, their findings could give insight into why antibody diversity declines with age and suggest interventions to address this to ensure improved health in later life.
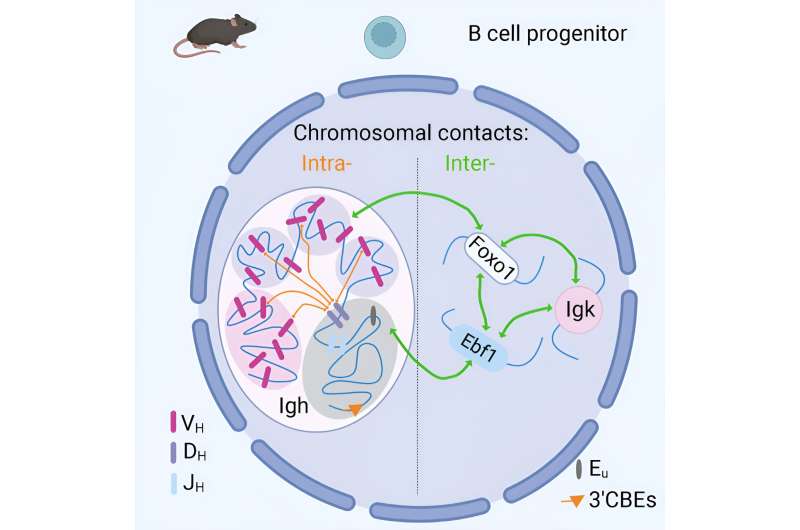
A multi-disciplinary team of immunologists, bioinformaticians, biophysicists, and pioneers in 3D genome analysis led by Dr. Anne Corcoran at the Babraham Institute have discovered that the 3D organization of DNA in B cells from mice allows for genes that are physically far away from each other to come together during antibody generation and generate the diversity of antibodies needed for robust protection against disease.
Surprisingly, they found that every B cell folds this part of the genome differently, rather than showing some conserved folding, meaning there are endless ways to combine genes into a unique order.
"We wanted to understand the mechanisms behind antibody variety. One way the cell achieves this is cutting and pasting from a suite of options for the antibody genes, but the puzzling thing is genes that are far away from the location of this event are used just as often as ones close by, so there must be some way of bringing everything together and making sure that everything needed is at hand," explained Dr. Anne Corcoran, senior group leader in the Institute's Immunology program.
Until now, researchers lacked the tools to investigate these mechanisms, but there were two schools of thought; one said the arrangement of DNA would be flexible and the other thought there would be common principles to the folding. Thanks to the Corcoran lab's refined methodology, they have been able to map the chromosome interactions in high resolution for the first time and resolve the debate.
The team developed a next generation sequencing method that allowed them to gather a deeper level of DNA configuration information from B cells. The technique was a development of the enriched Hi-C genome analysis method developed at the Institute which identifies points of direct chromosomal contact across the genome at high resolution.
With this enriched data set, they were able to understand more about the looping of the DNA and chromosomal interactions. The Babraham team collaborated with Dr. Luca Giorgetti's lab at the Friedrich Miescher Institute (FMI) in Basel, Switzerland, to generate 3D simulations of thousands of gene structures to validate their findings.
As well as mapping the interactions of the antibody genes, the team also found associations between key genes that support the development of B cells in a developmental stage-specific and cell type-specific way. The team suggest that these chromosomal contacts may be important for the correct and coordinated regulation of gene expression as the B cell develops.
"We found that the antibody genes were in close proximity to a small proportion of other genes on different chromosomes. Once we characterized them we found that these genes were associated with B cell development," Dr. Corcoran said, "and that was very interesting to see because if we apply this approach to other cell types we might be able to find important chromosomal interactions that are unique to those cell types."
The team speculate that the DNA folding might influence the number of different antibodies produced by the body as we age. During aging, our bodies make a smaller range of antibodies, as B cells select fewer genes from the available repertoire. In particular, they use fewer of the ones that are more remote.
It is possible that this is due to changes to the DNA folding mechanism that mean some genes become 'unreachable' and are no longer selected for antibody generation. The next steps for this research will be to investigate the 3D organization of antibody-specifying genes in aging B cells.
The work is published in the journal Cell Reports.
More information: Olga Mielczarek et al, Intra- and interchromosomal contact mapping reveals the Igh locus has extensive conformational heterogeneity and interacts with B-lineage genes, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.113074