This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Latest obesity drug not cost-effective for adolescents
Among today's obesity drugs, Wegovy (semaglutide) produces the greatest weight loss in teenagers, but a study by Columbia researchers has found that the trendy obesity drug is not cost-effective at its current price.
"All obesity medications on the market today are effective: they lead to weight reduction and improvements in health," says Chin Hur, MD, MPH, professor of medicine at the Vagelos College of Physicians and Surgeons and professor of epidemiology at the Mailman School of Public Health.
"But in the context of the U.S. health system, spending on semaglutide is not an efficient use of resources. The slight increase in weight reduction caused by semaglutide compared to a less expensive alternative (top-dose phentermine/topiramate) does not offset semaglutide's much higher cost when considering cost and effectiveness together."
Treatment for adolescent obesity
Obesity treatment with drug therapy and lifestyle counseling is now recommended by the American Academy of Pediatrics for adolescents ages 12 years and older with obesity, and several medications have been approved by the U.S. Food and Drug Administration for use in teenagers.
Wegovy, approved in Dec. 2022, was the latest to be authorized, following Saxenda (liraglutide, a drug that targets the same GLP-1 receptor as Wegovy) in July 2022 and Qsymia (a combination of phentermine and topiramate) in 2020.
Until now, no studies have compared the cost-effectiveness of obesity medications in adolescent patients.
"We felt this study was both timely and needed with the recent publication of results from the phentermine/topiramate and semaglutide adolescent clinical trials as well as their recent FDA approvals for adolescents," says the study's first author, Francesca Lim, MS, a research analyst on Hur's team within the Columbia Healthcare Innovation Research and Evaluation (HIRE) group.
Long-term data needed
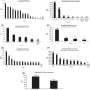
The new study projected the health and cost outcomes of four different obesity drugs—semaglutide, liraglutide, mid-dose phentermine and topiramate, and top-dose phentermine and topiramate—over 13 months, 2 years, and 5 years among a hypothetical group of 100,000 adolescents with an initial body mass index of 37.
Only top-dose phentermine/topiramate had an acceptable cost-effectiveness over 5 years when compared to lifestyle counseling alone. An intervention is generally considered cost-effective in the United States if its incremental cost-effectiveness ratio (ICER) is less than $100,000 per QALY gained. The new study calculated an ICER of $56,876 for top-dose phentermine/topiramate and $1.1 million for semaglutide.
The cost of Wegovy (about $1,400 per month without insurance coverage) would need to be reduced by 85% to match the cost-effectiveness of top-dose Qsymia, the study found.
Lim says the cost-effectiveness of the drugs evaluated in their study could change as more long-term data becomes available. The longest study of weight-loss drugs in adolescents only lasted approximately 1 year.
"We need data from studies that collect data for 10 to 20 years before we can fully understand the safety, efficacy, and cost-effectiveness of using these medications in adolescents," she says.
The study, titled "Cost-Effectiveness of Pharmacotherapy for the Treatment of Obesity in Adolescents," was published Aug 31 in JAMA Network Open.
More information: Francesca Lim et al, Cost-Effectiveness of Pharmacotherapy for the Treatment of Obesity in Adolescents, JAMA Network Open (2023). DOI: 10.1001/jamanetworkopen.2023.29178