This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New trial suggests that N-acetylglucosamine restores neurological function in multiple sclerosis patients
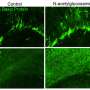
UCI researchers have found that a simple sugar, N-acetylglucosamine, reduces multiple inflammation and neurodegeneration markers in people who suffer from multiple sclerosis (MS). In addition, they also found this dietary supplement improved neurological function in 30% of patients.
According to the World Health Organization, MS affects more than 1.8 million people, and while there are treatments to prevent relapses and improve quality of life, there is no cure.
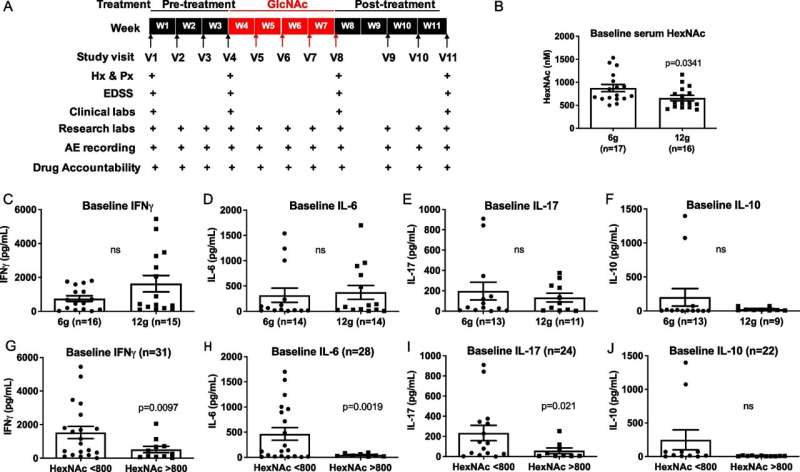
The study, N-acetylglucosamine inhibits inflammation and neurodegeneration markers in multiple sclerosis: a mechanistic trial, was published in the Journal of Neuroinflammation. Michael Demetriou, MD, Ph.D., Chief of the Division of Multiple Sclerosis and Neuroimmunology at UCI, is the lead investigator of the study. Michael Y. Sy, MD, Ph.D., Director of the Neuroimmunology Fellowship at UCI School of Medicine, is the first author, and Barbara Newton, MD, Project Scientist at UCI, is the second author.
A major issue with current therapies in MS is the inability to treat chronic-active neuroinflammation in the brain and the associated failure to repair the loss of myelin that covers and protects axons, the electrical wires of the brain. Over time, this leads to permanent nerve cell damage and slow progressive loss of neurological function in patients.
"Our previous studies in mice and humans implicated N-acetylglucosamine in suppressing brain inflammation, promoting the regrowth of the myelin sheath and slowing brain degeneration," said Michael Demetriou, MD, Ph.D., Professor of Neurology and Microbiology & Molecular Genetics at the UCI School of Medicine.
The new paper reports on the first clinical trial of N-acetylglucosamine in MS patients to directly investigate these potential activities. The trial was developed and performed exclusively in the Demetriou Lab at the UCI School of Medicine and UCI's Institute of Clinical and Translational Science.
Researchers found that N-acetylglucosamine was safe and reduced multiple inflammation and neurodegeneration markers in MS patients despite the patients already being on the FDA approved immunomodulatory therapy Glatiramer Acetate, known to impact these pathways outside the brain.
"We also observed a sustained reduction in neurological disability in 30% of the patients, an activity which has not been observed with current FDA-approved therapies," said Michael Y. Sy, MD, Ph.D., Associate Professor of Neurology, UCI School of Medicine. "They at best slow progression, not improve function."
The data suggest that N-acetylglucosamine reduced untreated chronic-active neuroinflammation and/or promoted myelin repair. However, the researchers stress that the trial was unblinded and therefore future blinded studies and additional parameters are essential to validate N-acetylglucosamine's potential to improve residual chronic-active brain inflammation, myelin repair, neurodegeneration and neurological function in MS.
"Future studies demonstrating that N-acetylglucosamine can restore neurological function in MS patients would be a gamechanger and provide something that no other current therapy can do," said Dr. Demetriou, MD, Ph.D.
More information: Michael Sy et al, N-acetylglucosamine inhibits inflammation and neurodegeneration markers in multiple sclerosis: a mechanistic trial, Journal of Neuroinflammation (2023). DOI: 10.1186/s12974-023-02893-9