This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Copycat nutrient leaves pancreatic tumors starving, may inform cancer treatment
A study led by scientists at Sanford Burnham Prebys suggests an entirely new approach to treat pancreatic cancer. The research shows that feeding tumors a copycat of an important nutrient starves them of the fuel they need to survive and grow. The method, described in the journal Nature Cancer, has been used in early clinical trials for lung cancer. However, the unique properties of pancreatic cancer may make the strategy an even stronger candidate in the pancreas.
"Pancreatic cancer relies on the nutrient glutamine much more than other cancers, so therapies that can interfere with tumors' ability to access glutamine could be highly effective," says senior author Cosimo Commisso, Ph.D., director and associate professor of the Cancer Metabolism and Microenvironment Program at Sanford Burnham Prebys.
Pancreatic cancer is relatively rare, accounting for only 3% of all cancers. However, it has one of the lowest survival rates among cancers: most people only live three to six months after being diagnosed with this disease.
"Over the course of the past decade, there has been a notable improvement in survival rates for pancreatic cancer, but they still hover around just 10%," says Commisso. "There is a dire need for new treatments for these cancers."
One of the challenges of treating pancreatic cancer has to do with the physical properties of the tumors themselves.
"Pancreatic tumors tend to be packed in dense connective tissue that keeps them encapsulated from the rest of the body and cuts off their supply of oxygen," says Commisso. "As a consequence, these cancers develop unique metabolic properties compared to other tumors, and this is something we may be able to exploit with new treatments."
One of the metabolic quirks of pancreatic cancer is that it relies heavily on glutamine to produce energy for growth and survival. In the past, scientists have tried to block access to glutamine to slow the growth of pancreatic tumors, but this is easier said than done.
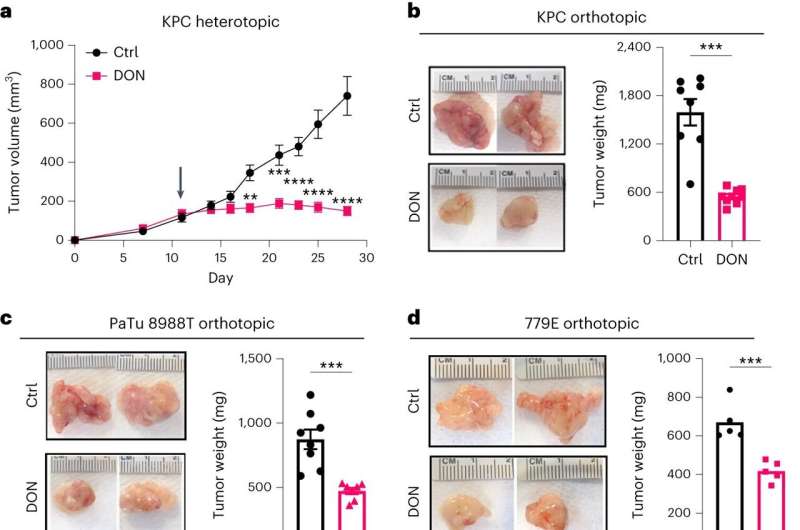
The new method relies on a molecule called DON that has structural similarities to glutamine but can't actually be used as a nutrient source. By studying mice, the research team found that DON significantly slowed pancreatic tumor growth and stopped the tumors from spreading.
Although DON was able to stop pancreatic tumors from using glutamine, pancreatic cancer cells can use other nutrients to grow in glutamine's absence. To combat this effect, the researchers combined DON with an existing cancer treatment that blocks the metabolism of asparagine, another important nutrient. The combined treatment had a synergistic effect, helping prevent the spread of pancreatic tumors to other distant organs, such as the liver and lungs.
"With DON, the cancer cells can't use glutamine, but they can start to depend on other nutrients as a backup, including asparagine," says Commisso. "We thought that if we could stop them from using glutamine and asparagine, the tumors would run out of options."
Although this is the first time this combination of treatments has been proposed for any cancer, the approach of using DON on its own has already advanced to early clinical trials in lung cancer.
"This is particularly exciting, because exploring it further for pancreatic cancer patients could be relatively simple, since the study designs exist for other solid tumors," adds Commisso. "This could be a game changer for pancreatic cancer, and a lot of the preclinical work needed to rationalize it is already happening."
More information: Maria Victoria Recouvreux et al, Glutamine mimicry suppresses tumor progression through asparagine metabolism in pancreatic ductal adenocarcinoma, Nature Cancer (2023). DOI: 10.1038/s43018-023-00649-1