This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Newfound mechanism suggests drug combination could starve pancreatic cancer
A new combination of treatments safely decreased growth of pancreatic cancer in mice by preventing cancer cells from scavenging for fuel, a new study finds.
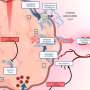
Led by researchers at NYU Grossman School of Medicine, its Department of Radiation Oncology, and the Perlmutter Cancer Center, the work builds on prior discoveries at NYU Langone that revealed how pancreatic cancer cells, to avert starvation and keep growing, find alternate fuel sources.
Normally supplied by the bloodstream, oxygen, blood sugar, and other resources become scarce as the increasing density of fast-growing pancreatic tumors cuts off their own blood supply. In this environment, the ability to switch fuels contributes to the deadliness of pancreatic cancers.
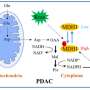
Published in Nature Cancer, the new study involves a drug designed to prevent pancreatic ductal adenocarcinoma (PDAC) cells from one such switch. PDAC cells use the enzyme glutaminase to convert the amino acid glutamate into glutamine, a form that can be burned for fuel to sustain rapid tumor growth. Drugs designed to block glutaminase, however, have been shown to cause cancer cells to switch to still other scavenging pathways.
For this reason, the field next looked at experimental treatments like DRP-104, designed by Dracen Pharmaceuticals, a new "prodrug" form of the compound 6-Diazo-5-oxo-L-norleucine (DON) that is preferentially activated within tumors to overcome toxicity issues seen with DON. DON was designed to starve cancer cells by mimicking glutamine, which unlike glutaminase blockers, broadly inhibits all metabolic pathways that use glutamine. Currently in clinical trials against non-small cell lung cancer, DRP-104 cannot be burned as fuel, but clings to the same enzymes as glutamine.
In the current study, DRP-104 treatment alone decreased PDAC growth in mouse models of pancreatic cancer. Importantly, the current team also found that PDAC cells pushed into metabolic crisis by DRP-104 increase signaling through a protein called extracellular signal-regulated kinase or ERK, to make up for their loss of glutamine metabolism. When the team combined DRP-104 with an existing drug that blocks the ERK signaling pathway, trametinib, it further improved survival in pancreatic cancer mouse models compared to DRP-104 treatment alone.
"Despite a decade of advances in understanding how cancer cells switch fuel sources, we have not yet effectively translated this into clinically relevant therapies," says corresponding study author Alec Kimmelman, M.D., Ph.D., the Anita Steckler and Joseph Steckler Chair of Radiation Oncology at NYU Langone Health.
"Broad antagonism of metabolic pathways with glutamine analogs may provide another mode of attack against these highly resistant tumor cells. The fact that such drugs are already being tested in the clinic makes us hopeful that we may finally see patient outcomes improve, if this approach proves to be effective in clinical trials."
Moving forward, the research team will seek to understand how glutamine antagonism impacts other adaptive nutrient scavenging mechanisms in pancreatic cancers and whether these could be targeted as well. The success of such approaches will depend on careful balancing of improved therapeutic efficacy and toxicity from the potential effects on normal tissues, Kimmelman adds.
More information: Targeting Pancreatic Cancer Metabolic Dependencies through Glutamine Antagonism, Nature Cancer (2023). DOI: 10.1038/s43018-023-00647-3