This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Uncovering the role of skin microbiome and immune response in cutaneous leishmaniasis
The parasitic disease leishmaniasis is found primarily in Central and South America, the Mediterranean region, the Middle East, and Central Asia and is transmitted to humans by a sand fly. An estimated 1.5 million new cases of cutaneous leishmaniasis—the most common form of the disease—occur each year. There is no vaccine, and treatment with anti-parasitic drugs is often unsuccessful.
People with cutaneous leishmaniasis can develop painful and disfiguring skin ulcers that, if left untreated, may metastasize to the nasopharyngeal region and turn into mucosal leishmaniasis, a more debilitating version of the disease that is untreatable.
Now, two new studies led by researchers at the University of Pennsylvania's School of Veterinary Medicine and the Perelman School of Medicine has shed light on the complex pathology of the parasitic disease leishmaniasis, pointing the way to potential new therapies.
"It's a horrific disease," says Phillip Scott, vice dean of Penn Vet and a longtime leishmaniasis researcher. Some years ago, Scott started collaborating with Dan Beiting, associate professor in Penn Vet's Department of Pathobiology to look at the transcriptional profile of the human disease.
This work was expanded to investigate the role of bacteria in the skin, and Beiting established a collaboration with Elizabeth A. Grice, an associate professor in Perelman's department of dermatology and an expert on the skin microbiome—the microorganisms on the skin. "We started collaborating because we realized that the skin microbiome might be influencing what's happening with the disease," Scott says.
It turns out they were right.
With the support of researchers in Brazil, they uncovered the pivotal role of the host's immune response to leishmaniasis, a pathologic response influenced by the presence of bacteria in skin lesions.
Their findings were published in Science Translational Medicine and the Journal of Experimental Medicine. "These two papers are probably the most impactful of [our] collaboration so far," Scott says.
The researchers found that it's often the host's immune response to parasitic infection, rather than the parasite burden itself, that promotes the most severe disease.
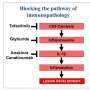
"You need a drug that not only controls the parasite," Scott says, "but, also one that limits a pathologic host inflammatory response. And so, we're very interested in trying to downregulate that inflammatory response to lessen disease."
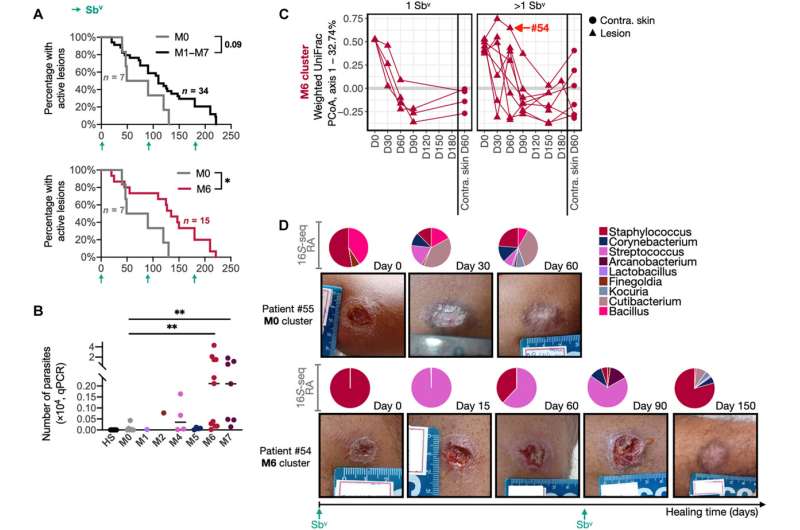
Scott and Grice also show that the skin microbiome plays a significant role in promoting those immunopathologic responses. Using a combination of transcriptional analysis and 16S sequencing in lesions from patients, they found that high levels of bacteria in those lesions—specifically, high levels of Staphylococcus aureus—are associated with treatment failure. These results suggest that therapies that reduce the S. aureus burden in leishmaniasis lesions might promote more rapid healing.
"People have been studying leishmaniasis for many years, and they've thought of it as being caused by one pathogen. It's not," says Scott "It is a disease caused both by the parasite and the increased bacterial burden in lesions."
Grice says, "The important takeaway here is that the microbiome can modify the severity of the infections and influence the disease outcomes. Our approach of studying human leishmania infections to identify markers of disease severity, and then taking those findings into model systems to study mechanisms is very powerful."
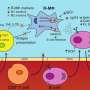
To understand the mechanisms involved in the increased pathology associated with bacteria, the researchers studied S. aureus infections in mice with leishmaniasis. They found that colonization of the skin with S. aureus led not only to more severe disease, but also to an increase in regulatory T cells, or Tregs. These cells are known to suppress immune responses and play a critical role in preventing autoimmunity.
To determine the role of Tregs in leishmaniasis, the researchers partially deleted them from the mouse models and found that those mice exhibited more severe disease compared to the mice with normal Treg numbers. Importantly, this increased disease was not associated with more parasites, but was associated with a large increase in the S. aureus burden.
To find out if the results in mice held true for human patients, they returned to their transcriptional analysis of human leishmaniasis lesions and found variability in Tregs within those lesions, as assessed by expression of the Treg transcription factor FOXP3. Significantly, they found that patients with a low expression of FOXP3 in lesions, suggesting fewer Tregs, exhibited delayed healing compared with patients with a high expression of FOXP3.
Unexpectedly, the researchers also found that IFN-gamma, a cytokine normally associated with protection in leishmaniasis, was elevated in patients who exhibited delayed healing. These results show that IFN-gamma can play a pathologic role in leishmaniasis when lesions are co-infected with S. aureus, and provide the foundation for new studies to uncover how IFN-gamma promotes more severe disease.
Scott says the investigators' findings carry a message. "When studying infectious diseases, we should study them understanding that there are often multiple pathogens involved," he says. "This is well understood in the gut but is also occurring in the skin."
The research has important implications for leishmaniasis treatment.
Scott notes that these results indicate that curing leishmaniasis will not only involve killing the parasite with drugs but also controlling a S. aureus infection. We never realized this before. But it's important, since S. aureus can spread and cause life-threatening infections.
To Grice, the research on leishmaniasis is important—and urgent. "I think about it in terms of helping a population that's burdened with this disease," she says. But she anticipates that population will grow: With the reality of climate change, the sand fly is expected to expand its geographical habitat. "This is going to affect a larger part of the global population," says Grice.
Add in the fact that many bacteria, including S. aureus, are becoming multi-drug resistant, she adds, and the need to identify alternative therapies becomes more pressing.
More information: Camila Farias Amorim et al, Multiomic profiling of cutaneous leishmaniasis infections reveals microbiota-driven mechanisms underlying disease severity, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.adh1469
Tej Pratap Singh et al, Regulatory T cells control Staphylococcus aureus and disease severity of cutaneous leishmaniasis, Journal of Experimental Medicine (2023). DOI: 10.1084/jem.20230558