This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Breakthroughs and challenges in fungal vaccine development
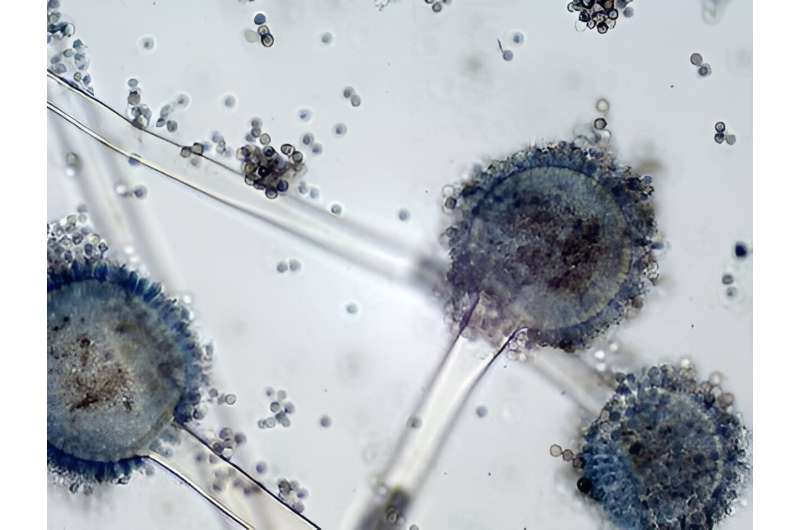
The microbiological world is comprised of many organisms, including bacteria, viruses, and fungi. Though not generally regarded as pathogens by the greater public, fungi can cause an array of severe diseases, especially in immunocompromised populations.
In Oct. 2022, the World Health Organization (WHO) released the WHO fungal priority pathogens list to highlight unmet research and public health policies required to address fungal disease as a growing global health concern.
The top three fungal pathogens on the list (Candida auris, Aspergillus fumigatus and Cryptococcus neoformans) are of major concern. C. auris, is a dangerous antifungal-resistant yeast that can appear on different areas of the body and turn lethal if it enters the bloodstream (systemic candidiasis). Meanwhile, fungal pathogens A. fumigatus and C. neoformans can cause severe pulmonary fungal infections resulting in invasive aspergillosis or cryptococcal encephalitis, respectively, in immunocompromised hosts.
The rise of antifungal resistance, immunosuppressive biomedical interventions and limited antifungal drugs have made clinical treatment of these fungal diseases increasingly difficult. In the U.S. alone, the estimated direct medical cost of fungal infections is $6.7-$7.5 billion annually.
While drug discovery is critical in the treatment of fungal infections, it is also challenging, given that fungi and humans share similar cellular machinery, unlike bacteria or viruses. What about creating a vaccine to prevent severe fungal disease from developing in the first place? Vaccine development is a major topic of research interest in the context of viral and bacterial disease, yet there are currently are no Federal Drug Administration (FDA)-approved fungal vaccines available for clinical use. So, what is needed to create a fungal vaccine?
What does a fungal vaccine need to prime an immune response?
One of the biggest hurdles to overcome in constructing a fungal vaccine the necessity to induce protection in immunodeficient conditions.
Immunocompromised populations (e.g., people with weakened immune systems, people who have had stem cell transplants or organ transplants, people who are receiving chemotherapy and/or have cancer, people with HIV, people taking immunosuppressants, etc.) have a significantly elevated risk of acquiring severe fungal infection.
The elevated risk and propensity for severe infection makes these populations the target demographic for a fungal vaccine.
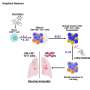
To mount a proper immune response against a fungal foe, different leukocytes (including macrophages, neutrophils, and monocytes) are needed to activate the adaptive immune response for long-term protection. An effective long term antifungal response requires different lymphocytes too, depending on the fungal pathogen and site of infection.
For example, in mucocutaneous candidiasis where Candida infects skin and mucosal membrane surfaces of the host, lymphocytes (TH17, CD8+, ILC3, and gdT cells) that produce cytokines (IL-17 and IL-22) are needed to fight infection. Whereas myeloid phagocytes (neutrophils, macrophages, monocytes) are required in protection against systemic Candida infection (invasive candidiasis).
In cryptococcosis, where inhaled Cryptococcus spores lodge in the lungs and disseminate as yeast to the blood brain barrier, TH1 CD4+ T cells are critical producers of key protective cytokines (i.e. IFN-g, IL-12, IL-2) that recruit and activate phagocytes to intracellularly kill phagocytosed cryptococci during infection.
Yet, in aspergillosis, a lung infection caused by Aspergillus mold (commonly A. fumigatus), CD4+ T cell involvement is a protective redundancy, and protection is mediated by myeloid cell (neutrophils, CCR2+ monocytes, plasmacytoid dendritic cells) crosstalk in the lung.
Differences in immunological responses against fungal pathogens are exacerbated by the fact that individuals most at risk for fungal disease are missing one or the other compartments of immunity (innate or adaptive). This makes generating a fungal vaccine for each or all combined ("pan-fungal" vaccine), which can operate in multiple types of immunocompromised settings, quite arduous. For now, generating a candidate vaccine that can enhance the under-developed arms of host immunity in immunocompromised conditions, or induce protection prior to immunosuppression, with high specificity to a common fungal antigen is crucial.
Fungal cell wall antigens are key
Since fungi and mammalian cells share similar cellular machinery, the fungal cell wall is a key aspect and great target for fungal vaccine development, as its structure/features are unique to fungal cells. Common to almost all fungal organisms' cell wall are a complex of crosslinked carbohydrate polymers β-glucans, mannoproteins, and chitin on top of the plasma membrane.
Thus, these components of the cell wall can act as viable immunogens to stimulate host immune responses. Promising vaccine candidates against Candida, Cryptococcus, Aspergillus and other fungal pathogens have focused on fungal cell wall antigens that can have enhanced immunogenicity when combined with adjuvants, overexpressed in whole cells or isolated and synthesized into peptides.
For encapsulated yeasts (i.e. Cryptococcus), a lipopolysaccharide capsule made up of galactoxylomannan (Gal-XM) and glucuronoxylomannan (GXM) mask the fungal antigens of the cell wall yet induce antibody production.
Other potential fungal vaccine targets are secreted fungal extracellular vesicles (EVs). Fungal EVs are comprised of bilayer phospholipid membrane particles that transport various proteins and nucleic acids outside of the cell where they can contribute to restructuring fungal cell wall in different stress conditions, alter host responses and modify biofilm formation.
Current fungal vaccine approaches
Live-attenuated or heat-killed whole cell vaccine
Many fungal vaccine candidates have demonstrated efficacy in murine models, but seldom have made it to clinical trials. Live-attenuated or heat-killed whole cell vaccines are methods of vaccination that have been tested against multiple fungal species. Essentially, these platforms work by introducing pathogens that have been attenuated or killed and are unable to cause infection. These strains still express immunogenic antigens present on or secreted by the parental virulent strains and initiate a protective immune response from the host upon subsequent challenge.
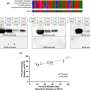
In C. neoformans vaccine models, multiple whole cell vaccine approaches have been identified to effectively protect against cryptococcosis infection in both immunocompetent and compromised settings. For example, an attenuated and whole cell Cryptococcus chitin deacetylase mutant strain (Cda1Δ2Δ3Δ) can confer protection against heterologous challenge with Cryptococcus by targeting chitosan formation, a process that is required for maintaining the cell wall structure.
While promising and successful in animal models, the underlying concerns with live-attenuated or even heat-killed whole cell vaccine approaches are the risks of infection in immunocompromised hosts and/or hyperinflammatory immune activation in autoimmune environments. These concerns have thus far precluded their testing in clinical trials and increased the search for alternate methods of vaccination.
Recombinant subunit peptide or protein sequences
Subunit peptide or protein sequences are an exciting vaccine methodology that may pave the way for future fungal vaccination. In this methodology, a purified immunogenic fungal antigen can be administered to the host in combination with an adjuvant to enhance immune activation. This method is generally considered a safer approach to vaccinating immunocompromised patients than live-attenuated vaccines because there is no pathogen involved to risk infection.
NDV-3A, a candidiasis vaccine candidate in phase II clinical trials, is a recombinant subunit vaccine comprised of agglutinin-like sequence 3 (Alsp3) combined with alhydrogel adjuvant that triggers T cell activation via IFNγ and IL-17 production and initiates antibody-mediated responses against Candida. Additionally, due to structural similarity between Alsp3 and Staphylococcus aureus surface proteins, this vaccine has been shown to be protective against S. aureus infection.
Future subunit vaccines will likely focus on being able to immunize against more than 1 strain at once due to antigenic variation.
Antibody based vaccines
So far, vaccination approaches have mainly focused on stimulated T cell activation of the immune response, since T-cell-mediated immunity is a predominant form of protection against a multitude of fungal pathogens. Some antibody-based vaccines have shown promise in combination with adjuvant and recombinant subunit fungal antigens, but with modest protection outcomes.
This method works by generating and isolating monoclonal antibodies made against specific antigen epitopes and combining them with adjuvants to boost the initial immune response upon vaccination. In one model of cryptococcosis vaccination, monoclonal Mab28 antibodies against β-glucan particles were able to reduce fungal burden and prolong survival in infection models, but they did not induce long term protection.
Commercialization and investment in fungal vaccines
Bringing a fungal vaccine to life as a preventative tool against fungal disease needs commercial investment if it is to ever become a reality. One of the largest concerns (and potential barriers) in generating interest from the biotechnology industry is the high cost of research for a product that will target a small percentage of the population (and diseases that largely affect low-income countries).
This may soon change as fungi are spreading to new environments due to climate change and the spread of antifungal resistance (most notably in C. auris). The cost associated with treatment of fungal infection and resulting hospitalization in the U.S. alone highlights the need for government investment in fungal vaccine research, and the economic burden will only increase without intervention.
Fungal vaccines are the future
Fungal pathogens are a global health concern that will persistently worsen if we do not invest and further antifungal vaccination development. Yet, despite the need, no fungal vaccine exists to date. To bring this critical tool to market, the following three points need to be addressed:
- Activating an effective antifungal immune response in at-risk, immunocompromised populations while mitigating undesirable side effects.
- Advocating for government agencies to incentivize investment in fungal vaccine research and commercialization as a public health policy initiative.
- Highlighting the economic impact a fungal vaccine will have as a preventative measure against fungal infections as climate change brings fungi to the forefront of environmental health.
The importance of continuing to enhance the effectiveness of current fungal vaccines, and discovering novel candidates, cannot be understated. Substantial fungal vaccine research has been done, but lack of government and biotech interest to commercialize is a major setback in the goal of generating a safe and effective fungal vaccine. We can change the tides of war against our fungal counterparts by prioritizing development of an effective vaccine to protect ourselves and the communities around us.