This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers identify cell signaling pathways controlling melanoma cell metastasis to the brain
Melanoma is the deadliest form of skin cancer because of its ability to quickly grow and spread throughout the body. More than half of those with advanced melanoma will see the disease spread to the brain, where it rapidly progresses, often leading to death in only three to four months. Researchers in Moffitt Cancer Center's Donald A. Adam Melanoma and Skin Cancer Center of Excellence have been working to better understand what drives melanoma brain metastasis.
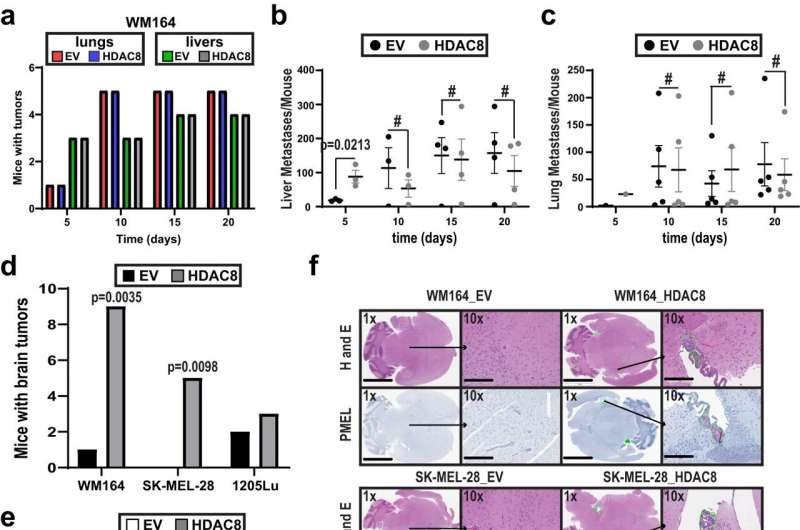
In a new study published in Nature Communications, they report on the identification of a cell signaling pathway that regulates the metastatic spread of melanoma cells to the brain.
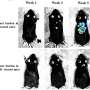
Melanoma tumors comprise subgroups of cells with different gene expression patterns with varied abilities to invade surrounding tissues and survive anticancer treatments. It is unclear how these different melanoma subgroups contribute to tumor development and progression.
In previous studies, Moffitt researchers determined that the protein HDAC8 regulated resistance to BRAF and MEK inhibitors commonly used to treat melanoma. HDAC8 removes chemical modifications called acetyl groups from other proteins, leading to alterations in gene expression patterns. The Moffitt team hypothesized that HDAC8 may also be involved in regulating gene expression patterns of melanoma cell subgroups.
The researchers performed laboratory experiments demonstrating that HDAC8 activity increased melanoma cell survival under stress conditions, including low oxygen, UV radiation, and BRAF/MEK inhibitor treatment. HDAC8 activity also changed the gene expression pattern of melanoma cells and caused the cells to develop characteristics associated with cell subgroups that could migrate into and invade surrounding sites.
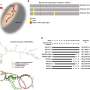
Their pre-clinical experiments found that increased HDAC8 expression and activity enhanced the ability of melanoma cells to metastasize to the brain, while no significant impact was observed in the number of metastatic tumors to other organs, such as the liver or lung. The researchers further investigated the molecular pathways of HDAC8-mediated brain metastasis and discovered that HDAC8 chemically modified the protein EP300, which subsequently caused cells to develop invasive characteristics.
The researchers confirmed the importance of EP300 to melanoma brain metastases by showing that increased expression of EP300 decreased cell invasion and caused melanoma cells to be more sensitive to cell death.
"These data show the importance of HDAC8 and EP300 activity to melanoma cell invasion to the brain and suggest that agents that target these pathways may inhibit brain metastasis," said Keiran Smalley, Ph.D., lead study author and director of Moffitt's Melanoma and Skin Cancer Center of Excellence. "Our work provides the first evidence that stress-induced HDAC8 is a regulator of an invasive melanoma cell state that leads to increased brain metastasis."
More information: Michael F. Emmons et al, HDAC8-mediated inhibition of EP300 drives a transcriptional state that increases melanoma brain metastasis, Nature Communications (2023). DOI: 10.1038/s41467-023-43519-1