This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Research connecting gut bacteria and oxytocin provides a new mechanism for microbiome-promoted health benefits
The gut microbiome, a community of trillions of microbes living in the human intestines, has an increasing reputation for affecting not only gut health but also the health of organs distant from the gut. For most microbes in the intestine, the details of how they can affect other organs remain unclear, but for gut resident bacteria L. reuteri the pieces of the puzzle are beginning to fall into place.
"L. reuteri is one of such bacteria that can affect more than one organ in the body," said co-corresponding author Dr. Sara Di Rienzi, assistant professor of molecular virology and microbiology at Baylor. "Researchers have found that these bacteria reduce gut inflammation in adults and rodent models, suppress bone loss in animal models of osteoporosis and in a human clinical trial, promote skin wound healing in mice and humans and improve social behavior in six mouse models of autism spectrum disorder."
Of those effects of L. reuteri, the abilities to promote social behavior and wound healing have been shown to require signaling by the hormone oxytocin, but little was known about how this occurs.
"We investigated the link connecting L. reuteri, oxytocin and distant organs such as the brain and uncovered unexpected findings," said first author Dr. Heather Danhof, assistant professor of molecular virology and microbiology at Baylor.
"Oxytocin is mostly produced in the hypothalamus, a brain region involved in regulating feeding and social behavior, as well as in other organs. Given that other brain-produced hormones also are made in the gut, we tested the novel idea that oxytocin itself was also produced in the intestinal epithelium where L. reuteri typically resides."
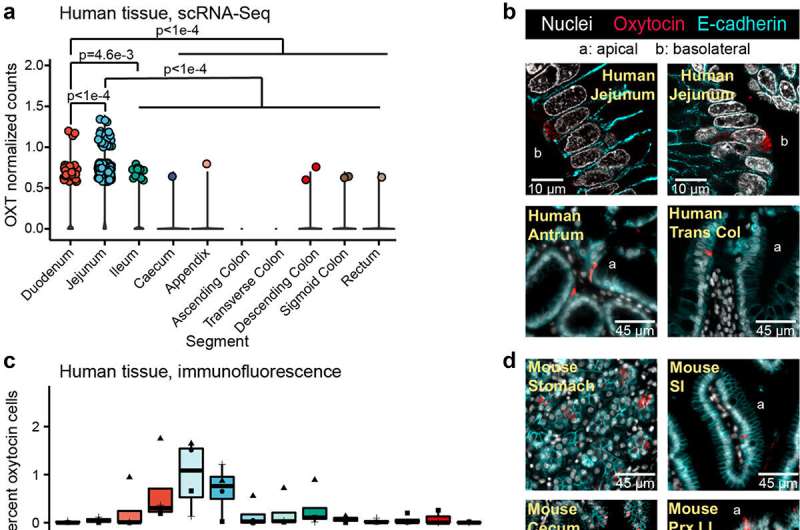
The researchers built up their case step by step. First, they reviewed single-cell RNA-Seq datasets of the intestinal epithelium, which show which genes are expressed in that tissue. They found that oxytocin genes are expressed in the epithelium of various species, including mice, macaques and humans. Then, using fluorescence microscopy, the team revealed the presence of oxytocin directly on human intestinal organoids, also called mini guts, which are laboratory models of intestinal tissue that recapitulate many of its functions and structure.
"Finally, a big moment was when we visualized oxytocin in human intestinal tissue samples, demonstrating oxytocin as an intestinal hormone," Di Rienzi said.
"We also determined a mechanism by which L. reuteri mediates oxytocin secretion from human intestinal tissue and human intestinal organoids," Danhof said. "L. reuteri stimulates enteroendocrine cells in the intestine to release the gut hormone secretin, which in turn stimulates another intestinal cell type, the enterocyte, to release oxytocin."
"We are excited about these findings," said co-corresponding author Dr. Robert Britton, professor of molecular virology and microbiology and member of the Dan L Duncan Comprehensive Cancer Center at Baylor.
"These bacteria have positive effects in various parts of the body, but it was not understood how that happened. Our findings reveal that oxytocin is also produced in the gut and a new mechanism by which L. reuteri affects oxytocin secretion. Now, we are working to identify potential treatments for autism spectrum disorders using a new mouse model deficient in intestinal oxytocin to gain a new understanding of the connection between oxytocin produced in the gut, social behavior and the brain."
The work is published in the journal Gut Microbes.
More information: Heather A. Danhof et al, Microbial stimulation of oxytocin release from the intestinal epithelium via secretin signaling, Gut Microbes (2023). DOI: 10.1080/19490976.2023.2256043