This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers detail mechanism of a key protein implicated in age-related brain dysfunction
Mount Sinai researchers have shed valuable light on the mechanism of a key protein that regulates the plasticity and function of the hippocampus, a key brain region involved in memory and learning, and that decreases with age in mice.
The team's findings, published in Molecular Psychiatry, could pave the way for a better understanding of how the protein, known as tissue inhibitor of metalloproteinases 2 (TIMP2), could potentially be targeted in age-related disorders like Alzheimer's disease to help restore affected molecular processes in the brain.
Aging is known to be the top risk factor for many neurodegenerative disorders, including Alzheimer's disease. Previous work by Mount Sinai researchers and others found that proteins that are enriched in young blood, including TIMP2, could be harnessed to rejuvenate brain function in aged animals by affecting plasticity—or the flexibility of neural processes related to memory—in the hippocampus. Despite that important discovery, little was known about the biology of how TIMP2 regulates plasticity of the hippocampus at the molecular level.
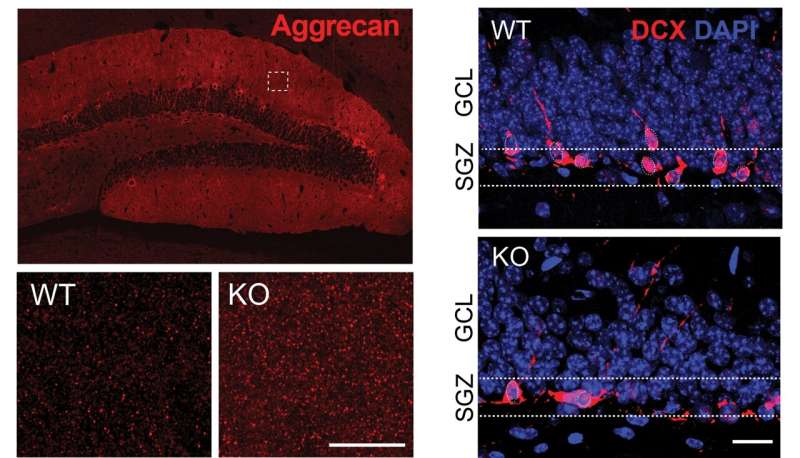
"In our latest study, we detailed a molecular link involving this protein that ties processes of plasticity, including the generation of new neurons in adulthood, to the structural nature—or what we call the extracellular matrix—of the hippocampal microenvironment," says Joseph Castellano, Ph.D., Assistant Professor of Neuroscience, and Neurology, at the Icahn School of Medicine at Mount Sinai and senior author of the paper.
"TIMP2 controls these processes by changing the flexibility of the microenvironment through components of the extracellular matrix. Studying pathways that regulate the extracellular matrix could be important for designing novel therapies for diseases in which plasticity is affected."
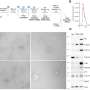
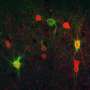
For their work, the team used a mutant mouse model mimicking the loss of TIMP2 levels in the blood and hippocampus that is known to occur with age. The team also created a model that allowed researchers to specifically target and delete the pool of TIMP2 expressed by neurons in the hippocampus. These models, in combination with RNA sequencing, confocal imaging, super-resolution microscopy, and behavioral studies, allowed for a detailed molecular examination of TIMP2's regulation of plasticity.
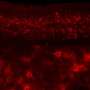
The researchers, including first author Ana Catarina Ferreira, Ph.D., a postdoctoral fellow in Dr. Castellano's group, learned that the loss of TIMP2 results in an accumulation of extracellular matrix components in the hippocampus that occurs alongside a reduction in plasticity processes, including the generation of adult-born neurons, synaptic integrity, and memory. The extracellular matrix is a network of many macromolecular components that make up the structural microenvironment around and between cells.
"We directly targeted this phenotype with an enzyme delivered to the hippocampus that affects the extracellular matrix and found that plasticity processes normally impaired in the setting of reduced TIMP2 were now restored," notes Dr. Castellano. "This finding has important implications for fundamentally understanding how plasticity is regulated at the structural level in brain regions involved in memory."
Overall, the findings suggest that targeting processes that regulate the extracellular matrix may be an important direction for designing approaches that improve plasticity in the brain. Dr. Castellano, whose lab is focused on characterizing factors with the potential to reverse features of brain aging, plans to explore molecules beyond TIMP2 that regulate the extracellular matrix, and is optimistic about where this research may take the field in the context of mitigating a variety of disorders associated with aging.
More information: Ana Catarina Ferreira et al, Neuronal TIMP2 regulates hippocampus-dependent plasticity and extracellular matrix complexity, Molecular Psychiatry (2023). DOI: 10.1038/s41380-023-02296-5