This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists piece together DNA repair pathway implicated in breast, ovarian and prostate cancers
Our DNA is not indestructible. Throughout the course of our lives, DNA can break in response to natural and environmental factors. Thankfully, our bodies have dedicated enzymes and pathways which can glue our broken DNA back together through several different mechanisms, known as DNA repair pathways.
Some cancers, however, can hijack these pathways for their own benefit. Susanna Stroik, Ph.D., and Dale Ramsden, Ph.D., both researchers in the Department of Biochemistry and Biophysics in the UNC School of Medicine and the UNC Lineberger Comprehensive Cancer Center, have pieced together the lesser-known DNA repair pathway, called polymerase theta-mediated end joining (TMEJ).
The pathway—which has been found to be upregulated in many patients with hereditary breast cancer, ovarian cancer, and prostate cancer, specifically those involving BRCA1 and BRCA2 mutations—has been laid out step by step in an article published in Nature, and the new knowledge could lead to new therapies for cancer.
"People with these breast cancer mutations, their cancers rely on polymerase theta's repair pathway to keep the tumors alive and repair DNA damage in the cancerous tissue," said Stroik, a postdoctoral researcher in Ramsden's lab. "Now that we know more about this pathway, scientists could, in theory, produce a drug that could disrupt key pieces of the pathway in cancer cells, as opposed to using conventional chemotherapies that destroy healthy cells along with the cancer."
Polymerase theta's discovery
Out of all DNA repair pathways, TMEJ has been the most elusive. Richard Wood, Ph.D., a distinguished professor at University of Texas MD Anderson Cancer Center played a key role in the first characterization of polymerase theta in 2003.
Over the next 15 years, multiple labs, including the Wood, Ramsden, and Gupta labs (also at Lineberger Comprehensive Cancer Center), were able to link polymerase theta to DNA repair (TMEJ) and cancer. Sylvie Doublié, Ph.D., an alumnus of UNC-Chapel Hill and professor of microbiology and molecular genetics at the University of Vermont, then solved the first structure of polymerase theta.
Together, and with other scientists from Penn State and New York University, these researchers were dedicated to understanding precisely what steps are involved in TMEJ, and which of those steps polymerase theta does and does not perform.
With the help of these collaborators, Stroik was able to use a wide variety of cutting-edge experimental approaches to fill in the gaps in our understanding of the TMEJ pathway. Critically, she discovered that another polymerase, called polymerase delta, uses a buddy system with polymerase theta to assist it in this repair pathway.
A unique buddy system
Stroik's research showed that polymerase theta is good at some things, but not others.
"It makes a lot of errors, and it's not capable of creating large swaths of DNA at once," said Stroik. "What was so beautiful and kind of elegant about the whole discovery is that there are two different enzymes alternating between pathway steps and helping each other out."
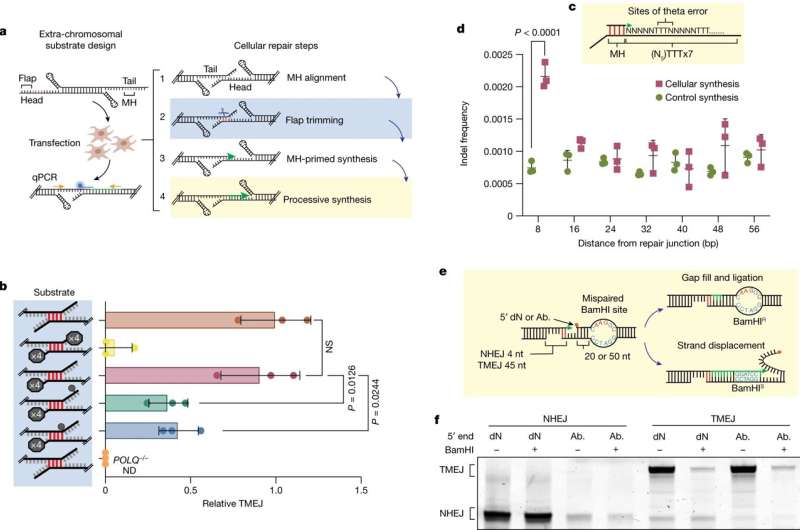
When a double-stranded break occurs, both strands of DNA are cut at the same spot, much like scissors severing a braid of hair. Polymerase theta acts quickly, grabbing the two single strands of DNA, matching up the closest base pairs to the break, and holding them together.
However, this often leaves some residual flaps of single-stranded DNA at the ends. Polymerase delta jumps in to cut the extraneous flaps, giving polymerase theta enough room to start synthesizing new DNA to fill in gaps in the DNA strands. Finally, polymerase delta jumps in one last time to help polymerase theta complete synthesis.
Stroik had another breakthrough finding: polymerases theta and delta are physically attached to one another. This new information could prove to be especially useful to drug developers hoping to create a new cancer treatment by drugging this interaction.
Cancer treatment potential
Since many cancers make use of the TMEJ pathway to keep tumors alive, many researchers have investigated creating drugs that can interfere with the pathway, essentially preventing cancer from repairing itself, leading to its eventual demise.
"Anytime you find new pieces of the pathway, you can 'drug' it," said Ramsden.
Stroik and Ramsden's new research will contribute to ongoing basic studies in polymerases theta and delta, while also aiding new cancer drugs called polymerase theta inhibitors, which are currently in clinical trials.
More information: Dale Ramsden, Stepwise requirements for polymerases δ and θ in θ-mediated end joining, Nature (2023). DOI: 10.1038/s41586-023-06729-7. www.nature.com/articles/s41586-023-06729-7