This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Team uncovers why maternal diabetes predisposes babies to heart defects
When women with diabetes become pregnant, they face not only the typical challenges of pregnancy and impending parenthood, but also a scary statistic: they're five times more likely to have a baby with a congenital heart defect.
Researchers at Gladstone Institutes have now discovered why that is, identifying the cells and molecules that go awry in the developing hearts of fetuses in women with diabetes. They found that a small subset of cells destined to become part of the heart's aorta and pulmonary artery have unusually high levels of retinoic acid activity, which coaxes them to behave more like cells found elsewhere in the heart.
The study, carried out in mice and published in Nature Cardiovascular Research, could eventually lead to interventions that lower the chance of heart malformations in babies born to women with diabetes. It also paves the way toward similar research on diverse array of other poorly understood birth defects.
"There are a number of environmental factors, including maternal diabetes, that we know are associated with birth defects, but we haven't been able to understand the mechanisms until now," says Gladstone President and Senior Investigator Deepak Srivastava, MD, the senior author of the new study. "This kind of modern, single-cell study can reveal these mechanisms and, ultimately, help us design therapeutic interventions to lower the risk of birth defects."
Srivastava is also the director of Gladstone's Roddenberry Stem Cell Center and a professor of Pediatrics and of Biochemistry & Biophysics at the UC San Francisco (UCSF) Medical Center.
Millions of heart cells
In mice and human embryos alike, millions of cells must respond to precise chemical signals, each at the right place and right time, to create a beating heart. If even a few embryonic cells receive the wrong molecular signals, the heart or nearby blood vessels can develop incorrectly, leading to congenital heart defects. However, due to the vast number and complexity of cells, pinning down what went wrong in any individual case of congenital heart disease is daunting.
"Congenital heart disease is the most common birth defect and comes with a huge social burden—it can be absolutely devastating for patients and their families," says Tomohiro Nishino, MD, Ph.D., a Gladstone postdoctoral scholar and first author of the study. "But without understanding the precise causes of these defects, there is really nothing we can do to prevent it."
Researchers do know that one of the most common non-genetic causes of congenital heart defects is maternal type I or type II diabetes before conception. These forms are distinct from gestational diabetes, which develops later in pregnancy, usually after a baby's heart has formed.
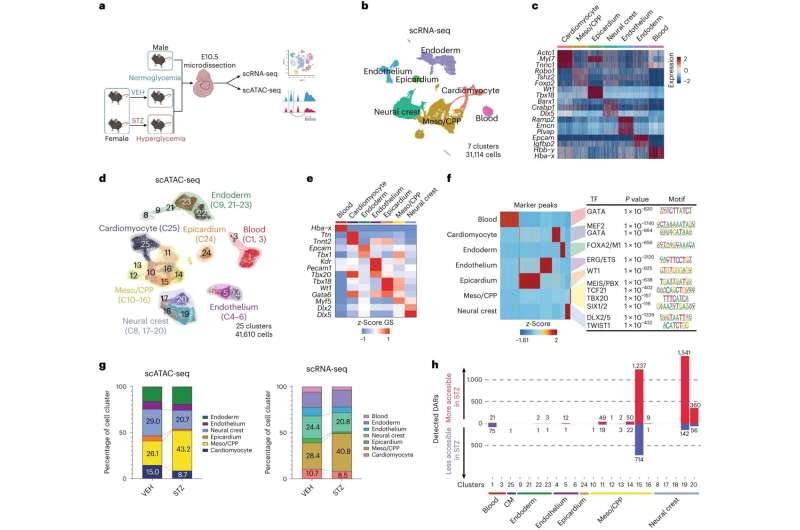
To learn how maternal diabetes contributes to heart defects, Srivastava's lab turned to a model of diabetic mice with high rates of heart defects in their offspring. The researchers collected more than 30,000 different cells from the developing hearts of embryos growing in the diabetic mice.
Then, the researchers analyzed both the three-dimensional configuration of DNA and the levels of different mRNA molecules in each individual cell, which encode proteins. Together, the experiments paint a picture of how genetic material is being used by the cells to dictate their functions.
"Coupling these two types of data let us determine not only how the cells are different when a fetus is exposed to maternal diabetes, but also what might be regulating that change," explains Srivastava.
A molecular trigger
When the researchers looked at how DNA was packaged into its tight, three-dimensional structure—which gives hints about which parts of the DNA molecule are being actively used by a cell—Srivastava's group found more than 4,000 differences between mice that are developing normally and those exposed to maternal diabetes.
Surprisingly, 97 percent of the differences were in two small subsets of cells, one of which goes on to form a critical section of the heart separating the aorta and pulmonary artery and chambers of the heart, and the other involved in facial development, another area affected in maternal diabetes. The subset of heart cells affected had higher-than-usual activity in a gene called ALX3, which controls the activity of many other genes.
"This subset of cells was never previously recognized as being different than the cells around it, so it was quite surprising to discover that those cells were so selectively vulnerable to maternal diabetes and responsible for the defects observed," Srivastava says.
The team went on to show that this subset of cells had high activity levels of the molecule retinoic acid, which is itself known to cause birth defects. Usually, higher levels of retinoic acid are found only in cells in the lower or posterior part of the heart-forming region. In maternal diabetes, the more anterior heart precursor cells that contribute to the aorta and pulmonary artery regions were being induced to behave more like more posterior cells, likely causing the observed defects.
More research is needed to determine how maternal diabetes changes levels of retinoic acid and why the newly discovered subset of heart cells is particularly susceptible to that increase. However the initial molecular understanding of what is triggering the heart defect offers a path forward.
The study also provides a template for using cutting-edge single-cell research to study links between environmental factors and birth defects more broadly. The same type of experiments could be used in other organ systems and for other exposures, such as drugs known to cause birth defects.
"The goal is eventually to have therapeutics that we can offer to mothers that lower their risk of all these birth defects," Srivastava says.
More information: Nishino, T. et al, Single-cell multimodal analyses reveal epigenomic and transcriptomic basis for birth defects in maternal diabetes, Nature Cardiovascular Research (2023). DOI: 10.1038/s44161-023-00367-y www.nature.com/articles/s44161-023-00367-y