This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Protective antibody targets conserved site of fusion glycoprotein of respiratory syncytial virus
Respiratory syncytial virus (RSV) is one of the leading pathogens that cause lower respiratory tract infections in infants and the elderly. Passive immunoprophylaxis with monoclonal antibody (mAb) has been approved to prevent morbidity and mortality from RSV infection in infants.
Palivizumab (Synagis) is an available humanized mAb against RSV infection and has been used prophylactically in premature infants or children with high-risk factors since 1998. However, limited efficacy, monthly dosing requirement, and high-cost restricted its clinical application and wide usage.
Therefore, development of a new generation of RSV mAbs with higher potency, longer half-life, and single-dose administration to cover the whole RSV season is a global priority.
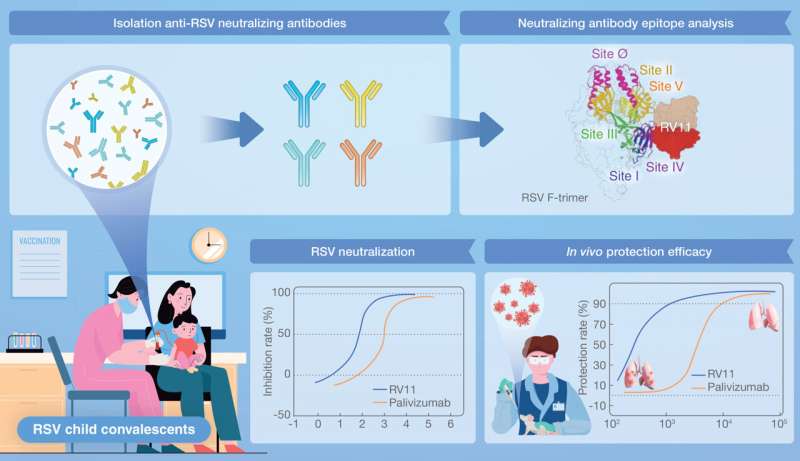
In a new study published in hLife, Dr. George Fu Gao (Institute of Microbiology, Chinese Academy of Sciences), Dr. Jim Zhen Wu (Shanghai Ark Biopharmaceutical), and Zhengde Xie (Beijing Children's Hospital, Capital Medical University) isolated two neutralizing mAb against RSV from convalescent children. One mAb RV11 exhibited good potency in neutralization in a cell-based assay. It protected mice from RSV infection, with ~6.3 higher efficacy compared to the commonly used Palivizumab.
This antibody binds to a highly conserved epitope on the fusion glycoprotein from both A and B genotypes of RSV. "This is very exciting!" Dr. Lianpan Dai says, "The good activity and highly conserved epitope of this antibody suggested its potential for clinical translation."
The team found the mAb RV11 is synergistic with ziresovir, a RSV small molecular inhibitor being considered for NDA in China, and a newly approved RSV mAb targeting a different site. The study also extends the knowledge to the neutralizing and protective epitopes of RSV.
More information: Lianpan Dai et al, A protective human antibody against respiratory syncytial virus by targeting a prefusion epitope across sites IV and V of the viral fusion glycoprotein, hLife (2023). DOI: 10.1016/j.hlife.2023.09.003