Monoclonal antibody against COVID-19 enters clinical trial
On June 5, 2020, China's National Medical Products Administration (NMPA) officially approved the clinical trials for a fully human monoclonal antibodiy (mAb) against COVID-19 developed by a research team at the Institute of Microbiology of the Chinese Academy of Sciences (IMCAS).
Phase I clinical trials will be carried out to test safety and appropriate doses in healthy human beings. This is also the first clinical trial in the world of therapeutic mAb in healthy people after evaluation in a non-human primate model.
Since mid-January, IMCAS has organized a number of research and technical teams to fight against COVID-19, including Prof. Yan Jinghua, and Gao Fu (George Fu Gao).
Dozens of fully human mAb genes were isolated and identified from convalescent patients of COVID-19. After repeated tests and comparisons, two ideally specific mAbs were screened out with highly effective neutralization of SARS-CoV-2 in late-February.
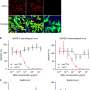
The researchers assessed the potencies of the mAbs with the rhesus monkey model. The results showed that the neutralizing mAbs effectively blocked the infection of SARS-CoV-2, significantly reduced the load of the new coronavirus in the respiratory tract of rhesus monkey, and protected against lung damage caused by virus infection.
COVID-19 has spread rapidly around the world, and the number of infected people has exceeded 6.6 million. Scientists all over the world are making efforts to find drugs effective against the virus, but there is still no specific drug approved.
Neutralizing antibodies are specific immunoglobulin types that target pathogens and blocks them from invading cells. The reported mAB represents an innovative specific drug developed for SARS-CoV-2. These clinical trials of mAbs have brought new hope to curb the spread of the virus.
The development of therapeutic mAbs is based on breakthroughs in the basic research on virus-host interaction. Prof. Yan and Gao, together with the researchers from Wuhan Institute Of Virology of CAS, PLA General Hospital, Center for Disease Control and Prevention, Capital Medical University, Beijing Ditan hospital and others, published a research paper on the fully human monoclonal neutralizing antibody against SARS-CoV-2 online in Nature, on May 26, 2020.
More information: Rui Shi et al. A human neutralizing antibody targets the receptor binding site of SARS-CoV-2, Nature (2020). DOI: 10.1038/s41586-020-2381-y