This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research sheds light on why brain implants lose functionality
Researchers have shed light on why brain implants are tricky to engineer and often lose their functionality once surgically placed into brain tissue.
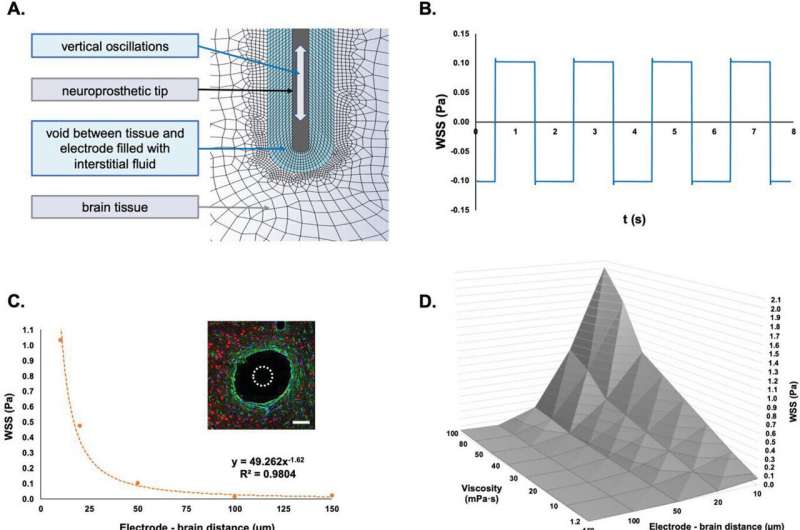
The results of a new study, published in Advanced Science, have revealed how cells of the brain sense continuous motion caused by everyday bodily functions—like breathing or the pulse from a heartbeat. Importantly, if a hard metallic or plastic device is implanted into the soft tissue of the brain, these small, normal movements can lead to friction and inflammation of the tissues around the implant, killing off vital brain cells and causing scarring.
The research was carried out by a team at CÚRAM, the Science Foundation Ireland research center for medical devices based at the University of Galway.
Lead researcher on the study, CÚRAM Investigator and Associate Professor at the University of Galway's College of Science and Engineering, Dr. Manus Biggs, said, "One of the most exciting parts of our study is the discovery that the cells of the brain use specialized sensors to respond to small frictional forces and that even the most basic, everyday functions can lead to tiny movements which damage the cells adjacent to a brain implant."
The research also explored possible approaches to help prevent damage to tissue and ultimately increase the lifespan and long term function of implanted electrical devices. Anti-inflammation approaches could be achieved by coating brain implants with soft gels which reduce implant friction and ensure a slow release of these drugs.
The study also evaluated how brain cells attempt to protect themselves from continuous friction by keeping a distance from hard brain implants, essentially creating a fluid-filled blister that prevents direct contact of an implant with the brain tissue. Although this blister which emerges around an implant protects the brain cells from damage, a frequent downside to this defense process is that this structure prevents the neural recording device from operating.
Dr. Alex Trotier, who carried out the principal research of the study at cúram and was awarded a Ph.D. by University of Galway, said, "Mitigating Scarring Of The Tissues Which Surround A Recording Device Implanted Into The Brain Is Critical For The Development Of Brain-Computer interfaces—devices which allow thoughts to be directly translated into digital signals, signals which can control external devices. The scar tissue that develops around an implanted neural device prevents brain signals from being recorded, rendering the device useless. The potential gamechanger here is for the development of digital implants which can read the brain electrical activity for years at a time."
Dr. Biggs added, "It is hoped that by understanding the cellular repair mechanisms, which occur following the introduction of a brain-implant, that novel devices or drugs can be developed which prevent the scarring and blistering process, paving the way for the emergence of exciting devices which can link the mind directly with advanced technologies. We may see the development of implants which can allow the instantaneous transmission of thoughts from one person to another in the next decade."
The research is published in the journal Advanced Science.
More information: Alexandre Trotier et al, Micromotion Derived Fluid Shear Stress Mediates Peri‐Electrode Gliosis through Mechanosensitive Ion Channels, Advanced Science (2023). DOI: 10.1002/advs.202301352