This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Understanding the risks of cell therapy for heart repair
A type of cell that plays a crucial role in tissue repair after a heart attack may also inadvertently be why cutting-edge cell therapies cause an increased risk of rhythm disorders, according to a new study from the Universities of Surrey and Oxford. Researchers hope the findings could open up new pathways to safe regenerative treatments for people who have suffered a heart attack.
The research focused on the interactions between cells created in the lab from stem cells called Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) with myofibroblasts, a type of cell that looks to repair heart tissue after a heart attack.
The study, published in Cellular and Molecular Life Sciences, found that myofibroblasts affect the electrical properties and calcium handling of hiPSC-CMs. Myofibroblasts also altered the expression of genes responsible for vital functions of heart cells, leading to electrical instability.
Dr. Patrizia Camelliti, lead author of the study from the University of Surrey, said, "Understanding the relationship between myofibroblasts and hiPSC-CMs could be the key to developing safe regenerative treatments for those who have suffered a heart attack. Our study identified Interleukin-6 (IL-6), a molecule released by myofibroblasts involved in inflammatory responses, as a key player in this interaction. We found that blocking IL-6 signaling reduced the negative effects of myofibroblasts on heart cells.
"While this study marks a step in the right direction for understanding how cell therapies unwittingly cause heart rhythm damage, further research is needed to bring these findings into clinical practice."
Heart attacks lead to the loss of heart muscle cells and the formation of scar tissue involving cells known as myofibroblasts. To repair this damage, scientists have been exploring the use of stem cells, such as hiPSC-CMs, to regenerate healthy heart muscles. However, these therapies have been shown to increase the risk of heart rhythm issues, which can be life-threatening.
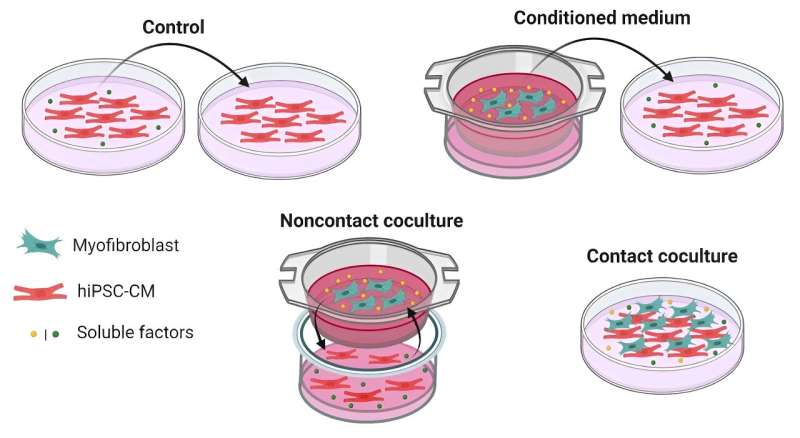
The research team used advanced cell culture systems to replicate the interactions between these cell types, observing the effects on heart cell function. They cultured hiPSC-CMs with adult human cardiac myofibroblasts in three conditions to mimic cell interactions: direct contact, non-contact, and medium conditioning.
Targeting interactions between cardiac cells could provide a novel therapeutic strategy to improve the outcome of cardiac cell therapies and treat heart rhythm disorders as discussed by Dr. Patrizia Camelliti in a perspective published in Science.
More information: Robert D. Johnson et al, Human myofibroblasts increase the arrhythmogenic potential of human induced pluripotent stem cell-derived cardiomyocytes, Cellular and Molecular Life Sciences (2023). DOI: 10.1007/s00018-023-04924-3
Patrizia Camelliti et al, Cellular coupling in the heart, Science (2023). DOI: 10.1126/science.adk3408