This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers discover deep structural biology connections that help improve CAR therapy
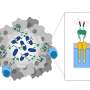
Chimeric antigen receptors (CARs) have opened up an exciting new field of therapeutic advancements for rare and difficult-to-treat cancers, as they have the ability to deliver targeted therapies that can kill tumor cells.
Peptide-centric CARs (PC-CARs) rely on specific peptide "barcodes," which are derived from proteins within the cell created by potentially cancer-causing oncogenes, are designed to find and target cancer cells. These "barcodes" are displayed by human leukocyte antigens (HLAs), which help the immune system distinguish its own proteins from foreign invaders, like viruses.
However, HLAs are derived from the most "polymorphic" genes, with more than 25,000 alleles—stretches of DNA that code for proteins that carry out essential functions—that could vary between them, making it difficult to design PC-CARs that target the specific alleles associated with different cancers.
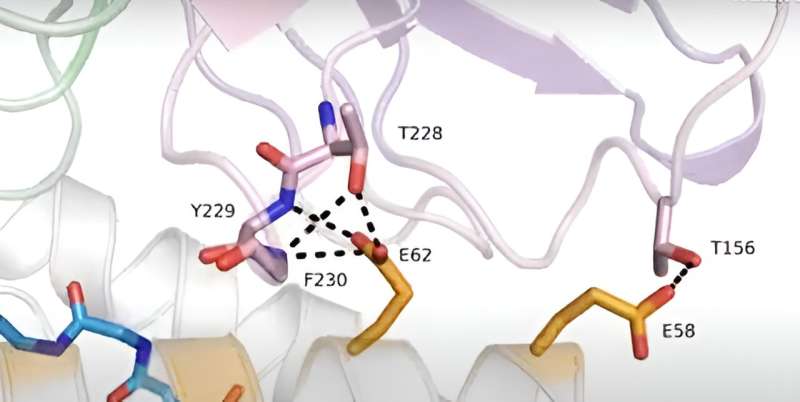
Now, researchers from two closely collaborating labs at Children's Hospital of Philadelphia (CHOP) have solved a three-dimensional protein structure that explains how PC-CARs can recognize the "backbones" of these HLA complexes. The structural information will now allow researchers to understand how CARs recognize tumor-associated antigens among different polymorphic HLA alleles, thereby opening up more possibilities for designing precision medicine strategies for more complex and difficult-to-treat tumors.
The findings were published online in the journal Science Immunology.
"If CARs are not matched properly to target the specific alleles associated with certain cancers, there is a risk of inducing toxicity without providing any therapeutic benefit," said senior author Nikolaos G. Sgourakis, Ph.D., Associate Professor in the Center for Computational and Genomic Medicine at CHOP. "By looking at their 3D complex structures, we can use these findings to design CARs that are able to target multiple HLAs and increase the efficiency of therapeutic design."
Earlier CAR therapies could only target cancer-specific antigens on the surface of tumor cells, and most of these reside within the cells. However, researchers have found that these previously inaccessible targets are eventually degraded into peptides, which can be expressed on the surface like "barcodes" and then targeted by therapy. Even then, with so much variability in the alleles of the HLA, CAR therapies might only be able to help a fraction of patients with tumors, depending on which peptides are expressed on the surface of a tumor cell.
Since HLAs have more than 25,000 potentially mutated alleles, going through them one-by-one to find potential targets and design associated CAR therapies is far too complex a task.
In this study, however, researchers used a combination of biochemical binding assays, molecular dynamics simulations and structural and functional analyses to determine that certain classes of HLAs are cross-reactive, meaning that different antigens can be similarly recognized by PC-CAR therapy. In other words, while the peptide "barcodes" may have significant variability, the "backbone" of these HLAs is similar enough to be recognized by these therapies.
This work was carried out by a group of undergraduate and graduate students from the University of Pennsylvania and CHOP senior scientists from the Sgourakis Lab.
CHOP has been a pioneer in the development of PC-CARs. Senior study co-author John M. Maris, MD, pediatric oncologist and Giulio D'Angio Chair in Neuroblastoma Research at CHOP, concurrently published an updated paper in the journal Nature about the development and effectiveness of PC-CARs, and patients are currently being recruited for clinical trials based on their HLA genotypes to further explore the effectiveness of PC-CARs for treating rare and complex forms of cancer.
"For any CAR T cell therapy to be both safe and effective, one must find 'targets' for the T cells to find the tumor. PC-CAR T cells hone to very specific targets that are only on cancer cells and not on normal healthy cells," Maris said.
"This study essentially provides us with a blueprint for how to integrate new knowledge about the structural biology of HLAs as well as PC-CARs in this exciting field of new options for treating challenging cancers."
More information: Yi Sun et al, Structural principles of peptide-centric chimeric antigen receptor recognition guide therapeutic expansion, Science Immunology (2023). DOI: 10.1126/sciimmunol.adj5792. www.science.org/doi/10.1126/sciimmunol.adj5792