This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study finds Minnesota connected more than 31,000 patients to monoclonal antibody treatments during the pandemic
Before the development and distribution of COVID-19 vaccines, monoclonal antibodies (mAbs) were considered one of the most effective and widely used therapies to reduce the risk of progression to severe COVID, especially for high-risk patients. Lab-created mAbs seek out foreign materials in the body, such as the COVID-19 virus, and stick to them in order to destroy them.
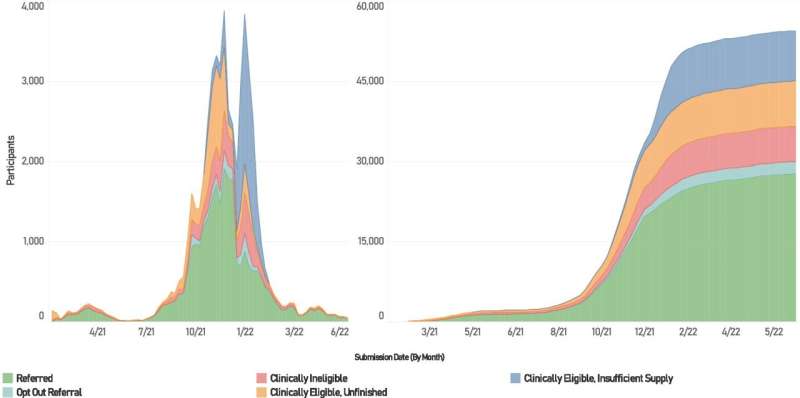
New research, published in Frontiers in Public Health, from the University of Minnesota School of Public Health (SPH) and several partners traces the development, implementation and health outcomes of Minnesota's system for distributing mAbs during the COVID-19 pandemic. According to the study, Minnesota's online platform connected more than 31,000 patients to care during its operation, successfully distributing a potentially life-saving treatment to those most eligible to receive treatment—regardless of their connection to a health care system.
A collaboration between the Minnesota Department of Health, the University of Minnesota and 116 private health care administration sites across the state developed an online platform, Minnesota Resource Allocation Platform (MNRAP), which facilitated access to mAbs when supplies were sufficient. When supplies ran low, MNRAP managed the allocation of resources by prioritizing those most at risk for hospitalization or death.
By analyzing the health outcomes of the people who interacted with MNRAP between February 2021 and July 2022, the study found that there was a statistically significant difference in hospitalizations for people who did not receive a referral through MNRAP versus those who did (5.2% for unreferred versus 6.1% for referred), because those who received referrals were at much higher clinical risk than those that did not.
It also found that vaccination conferred a substantially larger protection against hospitalization and death than did a referral for mAbs, but among unvaccinated users that did not get a referral, chances of hospitalization increased by 4.1%.
"In just a year and a half, MNRAP connected over 30,000 Minnesotans battling COVID-19 with monoclonal antibodies, regardless of whether they had a usual source of care," said JP Leider, SPH associate professor, lead author of the study, and U of M lead on the MNRAP project. "While vaccination proved to be the most protective against severe clinical outcomes, it appears monoclonal antibodies helped protect unvaccinated individuals against particularly bad outcomes."
Ensuring equitable distribution of treatment was a core mission of MNRAP. Even if supply was sufficient to meet demand, a process was needed to avoid unfairly disadvantaging patients whose health systems elected not to offer mAbs or patients who were not affiliated with any health system. MNRAP was able to clinically prioritize those most at risk for hospitalization or death, including operating a statewide lottery during deepest scarcity.
"Clinical risk factors should drive allocation decisions, not a patient's individual circumstances—such as access to health care or the resources and ability to navigate a complicated health system," says Debra DeBruin, director of the University's Center for Bioethics.
"Experience nationwide—documented in countless news articles about patients calling dozens of providers or needing to 'be connected' to find appointments—suggested patients might have had uneven access to this critical resource. That is exactly what MNRAP was designed to avoid."
More information: Jonathon P. Leider et al, Using a web platform for equitable distribution of COVID-19 monoclonal antibodies: a case study in resource allocation, Frontiers in Public Health (2023). DOI: 10.3389/fpubh.2023.1226935