This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
reputable news agency
proofread
Could a monthly treatment prevent fentanyl overdoses? Scientists are working on it
Scientists have developed an antibody treatment that shows promise in blocking the potentially deadly effects of fentanyl for nearly a month, raising hopes for a new tool to combat overdoses.
Tests in animals found that the treatment could effectively block the effects of fentanyl, laying the groundwork for assessing whether the medication will prove effective in humans, according to a study published in the journal Nature Communications.
The antibodies are too big to cross the blood-brain barrier, so when they bind to fentanyl in the bloodstream, they stop the powerful opioid from reaching receptors in the brain, researchers explained. The experimental treatment, dubbed CSX-1004, was administered to animals via an intravenous infusion.
Andrew Barrett, chief scientific officer for Cessation Therapeutics, the company that developed the treatment, likened the way it works to "Pac-Man" snapping up fentanyl in the blood, which "prevents fentanyl from ever getting to the brain where it produces its effects," both pleasurable and dangerous.
Among its possible uses: The infusion could be given as a preventive measure to patients who complete an inpatient detoxification program "to try to prevent a death in the event of a relapse," Barrett said.
Finding such a tool has been especially urgent as the number of American lives lost to drug overdoses has climbed to more than 100,000 a year, according to federal data. The bulk of those deaths have been linked to synthetic opioids such as fentanyl, a potent drug that has also been found in counterfeit pills and is often mingled with other drugs such as methamphetamine.
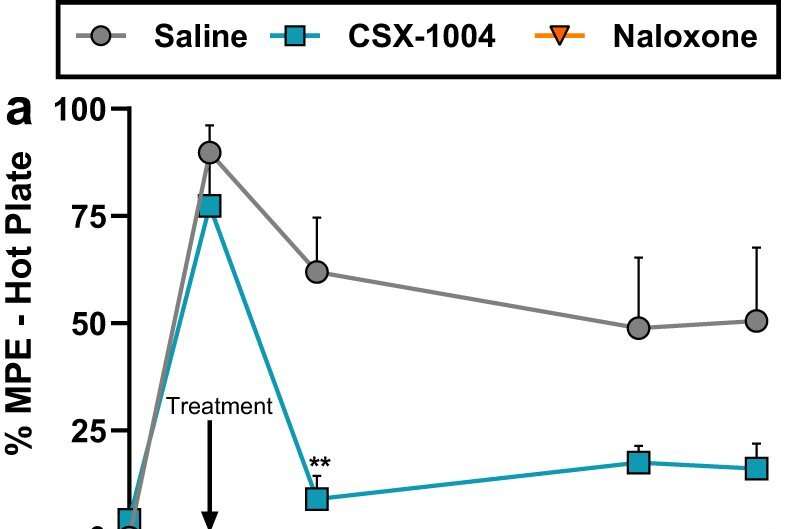
Scientists at Cessation Therapeutics in San Diego, teaming up with researchers from Harvard Medical School and McLean Hospital in Massachusetts, performed a series of tests in rodents and squirrel monkeys to gauge CSX-1004's effects and found that a single infusion protected the primates from fentanyl for more than three weeks.
One set of experiments included eight monkeys who were given fentanyl multiple times over 28 days. The animals first received a placebo to see how they responded to the opioid without treatment. Later, they received either a low or high dose of CSX-1004 at the outset of the cycle and were given fentanyl six times.
Breathing measurements showed the medicine was most protective for the first week and waned steadily after that, with the higher dose showing significant effects for as long as 28 days.
The experimental treatment reversed slowed or shallow breathing, an overdose symptom that can lead to death, researchers reported. And it worked on other dangerous fentanyl analogs like carfentanil—a synthetic opioid intended for sedating large animals— without affecting oxycodone or other opioids prescribed by doctors for pain management, the study authors found.
Cessation Therapeutics has started assessing the safety of the treatment in human volunteers and plans to next gauge its effects in people with opioid dependency, Barrett said. If CSX-1004 proves effective in clinical trials, the researchers said, it could protect people who are at high risk of overdose and become a bridge to other treatments that address craving and withdrawal symptoms, researchers wrote.
"We're in that crisis situation where we need all hands on deck to help prevent people from dying," said Kelly E. Dunn, a professor of psychiatry and behavioral sciences at the Johns Hopkins University School of Medicine who advised the company on the design for human trials of the medication but was not involved in data collection for the new study.
"These transformative approaches are exactly what we need," because with existing tools, "we're just not making the headway that we would hope."
Arming people with antibodies has long been explored by researchers as a potential way to protect people from a range of addictive drugs.
With fentanyl deaths up sharply since the start of the COVID-19 pandemic, the research on antibody treatments "really opens a door for a tool that will help us address very high-risk populations," said Dr. Nora Volkow, director of the National Institute on Drug Abuse. Among them are people exiting jails or prisons, who face an alarmingly high risk of overdose in the weeks after they are released, she said.
Volkow added that the treatment could help protect people who use cocaine or methamphetamine, who are in danger of overdosing when their drugs are contaminated with fentanyl. For those people, she said, "you will dramatically reduce the risk for dying."
The antibodies could have advantages over extended-release naltrexone, a medication that blocks the effects of opioids, Barrett said. Monthly injections of naltrexone require people to abstain from opioids for a while before beginning the medication.
"Your tolerance goes down very quickly" in that time, said Dr. Melissa B. Weimer, an associate professor of medicine and public health at Yale who was not involved in the study. As they undergo withdrawal, "most people who have severe opioid use disorder will be quite uncomfortable during that period of time. That places them at risk to not start treatment," leaving them unprotected and at higher risk of opioid overdose.
In addition, people taking naltrexone cannot use other common medications to reduce opioid cravings such as methadone. And because naltrexone blocks all opioids, it can complicate pain management in emergencies. Based on the findings so far, CSX-1004 doesn't share those drawbacks, Barrett said.
The research team is still studying whether CSX-1004 causes "precipitated withdrawal"—an abrupt and sickening experience that people have suffered with some treatments for opioid use disorder. "We don't know the answer yet, but we don't think it will be a profound withdrawal" because of the way it works, Barrett said.
Weimer said that another important question is what happens if people use more fentanyl to try to override the effects of the medication. Physicians will need to know if people could be at higher risk of overdose as the effects of the medication wear off, she said.
And then there is the practical question of "what proportion of individuals would opt for this treatment," said Dr. Larissa Mooney, an addiction psychiatrist at UCLA. "People who choose this therapy option would have to be motivated to receive a treatment that will reduce the effects of fentanyl."
Scientists have also tried to develop vaccines that prompt the body to produce antibodies that can protect against fentanyl and other illicit drugs. Dr. Thomas Kosten, a psychiatrist at Baylor College of Medicine who has been working on a fentanyl vaccine, said antibody-based therapies might have some practical drawbacks.
The cost of making monoclonal antibodies like CSX-1004 is "rather high," Kosten said, and "you can't just store a monoclonal in your closet, you have to refrigerate it. ... So in some ways, it's not a practical solution to what's a huge problem." He also questioned whether health insurers would cover the costs of an infusion treatment for substance use disorder.
Volkow said that if a treatment like CSX-1004 proves effective in humans, "there is a lot of room for innovation" to address practical issues. "It would make sense to do that innovation if it does look like it's going to work."
Cessation Therapeutics said it is already developing an injectable version of the antibody treatment that could be more convenient. In the new paper, the authors wrote that monoclonal antibodies could be priced around the same level as some existing treatments for opioid use disorder.
Dunn of Johns Hopkins University urged more investment in new medications and approaches to thwart overdoses. There is "very little general investment in this area," she said, despite the alarming number of lives lost. "We need that investment to be able to really make big changes."
More information: Paul T. Bremer et al, Investigation of monoclonal antibody CSX-1004 for fentanyl overdose, Nature Communications (2023). DOI: 10.1038/s41467-023-43126-0
2023 Los Angeles Times. Distributed by Tribune Content Agency, LLC.