This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Off-label use of a common antibiotic to treat muscular dystrophy
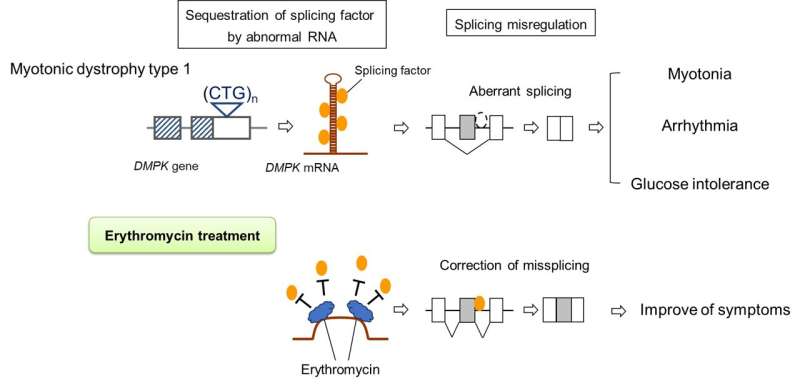
Myotonic dystrophy type 1 (DM1) is a genetic disease characterized by progressive muscular weakness. There is currently no treatment despite many recent efforts. But now researchers from Japan may have found a cure.
In a study published in eClinicalMedicine, researchers from Osaka University have reported an innovative approach to identify a possible new treatment for DM1, which has just passed phase 2 clinical testing with flying colors.
DM1 is the most common form of muscular dystrophy. It is caused by a genetic mutation that affects an essential process known as alternative splicing, which is important for correctly assembling proteins all over the body. In this way, DM1-causing mutations affect many different bodily systems, leading to a wide range of symptoms that are difficult to treat.
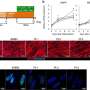
To identify potential therapies for DM1, the research team used drug repositioning screening, a method in which drugs that are already used for a specific purpose are tested for their effects on other diseases. They found that erythromycin—which you may already be familiar with, given its widespread use as an antibiotic—may be effective in DM1 because of its potential for improving splicing abnormalities.
"After our successful preclinical trials in DM1 cell and mouse models, we had high hopes for erythromycin," says Masayuki Nakamori, lead author of the study. "As the next step in getting this drug to the clinic, we conducted a trial on 30 people with DM1 to study the treatment's safety and tolerability, with a secondary aim of investigating its efficacy."
The researchers gave 6 of the 30 patients a placebo, while 12 received a low dose (500 mg) and 12 received a high dose (800 mg) of erythromycin for 24 weeks. Because these long-term doses are already used for other diseases, the researchers hoped that the safety data would be positive—and fortunately, they were right.
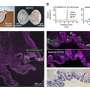
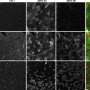
"We were also very interested in testing the efficacy of erythromycin in patients with DM1," explains Masayuki Nakamori. "To do this, we mainly looked at markers of aberrant splicing directly in patients' muscle biopsies."
Although this efficacy testing revealed few significant results, the research team noted that several patients who received erythromycin showed major improvements in splicing markers, suggesting that some patients respond better than others. Further analysis also revealed that some splicing markers were significantly improved at one dose of erythromycin but not the other, indicating that dosage may be important.
Despite the small sample size, the researchers are very optimistic. Erythromycin continues to be a promising therapeutic option, and subsequent phase 2b and 3 trials should be performed as soon as feasible to see whether this widely used antibiotic can successfully treat DM1, or at least a subset of patients.
More information: Masayuki Nakamori et al, Erythromycin for myotonic dystrophy type 1: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial, eClinicalMedicine (2023). DOI: 10.1016/j.eclinm.2023.102390