This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Organoids study discovers promising treatment for deadly uterine cancer
QUT scientists have discovered a promising new therapy for a deadly type of endometrial cancer that has a poor prognosis if the cancer spreads or returns after initial treatment, a plight that affects 15%–20% of endometrial cancer patients.
Dr. Asmerom Sengal and Associate Professor Pamela Pollock from QUT's School of Biomedical Sciences, published their research, "Endometrial cancer PDX-derived organoids (PDXOs) and PDXs with FGFR2c isoform expression are sensitive to FGFR inhibition," in npj Precision Oncology with a recommendation that the strength of their findings indicated they should proceed to patient trials.
Dr. Asmerom said endometrial cancer confined within the uterus could be cured with surgery however, if it had spread to the abdomen and other organs patients had limited treatment options.
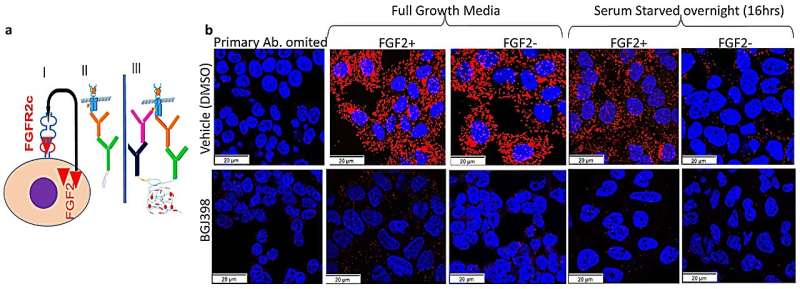
"Previously, we found women with endometrial cancer who have an incorrect growth factor receptor called fibroblast growth factor receptor 2c (FGFR2c) on the tumor cell surface have a poor survival rate," Dr. Asmerom said.
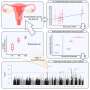
"For this study, we developed organoids—three-dimensional miniature tumors grown from patients' endometrial cancer cells in a hydrogel to enable us to study the complexity of the tumor structure and genetics.
"We found that endometrial cancer organoids with activated (or turned on) FGFR2c were blocked with an FGFR inhibitor drug and the organoids' growth was shattered and they died.
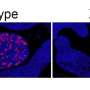
"These findings were further validated by blocking of 'turned-on' FGFR2c in patient-derived xenografts (PDXs)—endometrial cancers implanted in mice—with the same FGFR2c inhibitor which resulted in significant tumor growth inhibition, and the treated mice showed a remarkable increase in survival.
Dr. Asmerom said an interesting finding was that the endometrial cancer patient xenografts which harbored activated FGFR2c were treated with FGFR inhibitor for seven days and demonstrated remarkable reduction in tumor blood vessel formation and the immune cells (M2-macrophages) that prevent our immune system from killing cancer cells.
"Collectively, the study supports the initiation of a clinical trial combining a FGFR inhibitor with immunotherapy and this opens a new opportunity for personalized care in women with deadly endometrial cancer," he said.
More information: Asmerom T. Sengal et al, Endometrial cancer PDX-derived organoids (PDXOs) and PDXs with FGFR2c isoform expression are sensitive to FGFR inhibition, npj Precision Oncology (2023). DOI: 10.1038/s41698-023-00478-6