This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New findings reveal important insights into age-related nonresolving inflammation
Aging is associated with chronic, nonresolving inflammation, or "inflammaging," that can lead to tissue dysfunction. New findings reported in The American Journal of Pathology reveal insights into the cellular programs and factors that promote the resolution of inflammation during aging. These findings may lead to the development of new strategies to limit age-related organ decline.
The resolution of inflammation is an active process that is governed by numerous factors, such as specialized proresolving lipid mediators (SPMs). Recent studies suggest that inflammaging may persist due to an impairment in inflammation-resolution programs and that treatment with SPMs, like resolvins, tempers excessive inflammation and age-related tissue dysfunction.
Co-lead investigators Gabrielle Fredman, Ph.D., The Department of Molecular and Cellular Physiology, Albany Medical College, and Katherine C. MacNamara, Ph.D., The Department of Immunology and Microbial Disease, Albany Medical College, explained, "We realized early on in our collaboration that mechanisms associated with inflammation-resolution in aging were vastly underexplored. So, we combined our collective expertise to tackle some gaps in this arena."
To explore SPM-initiated mechanisms that limit features of inflammaging, researchers conducted a novel series of studies using the ligand, or chemical messenger, Resolvin D2 (RvD2). RvD2 acts via a specific G-protein–coupled receptor called GPR18, which investigators found was associated with maintaining tissue homeostasis during aging.
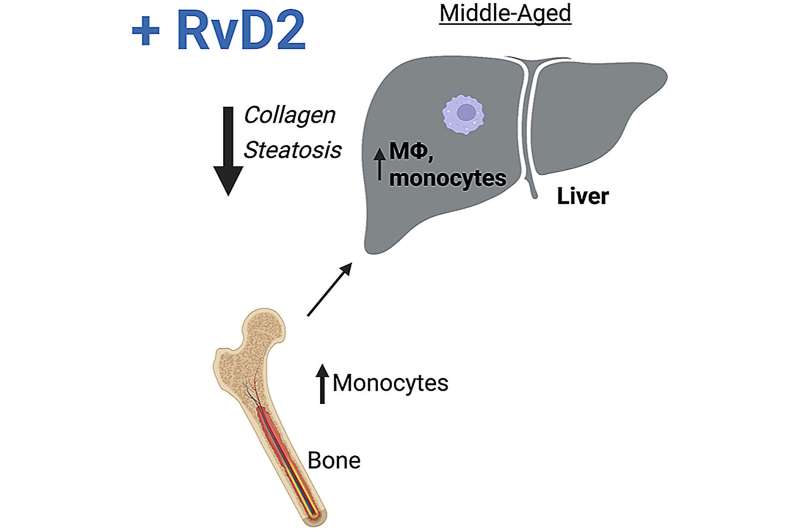
Using mice to model normal, healthy aging, investigators identified key pathologic changes in the liver that occur in middle age, including steatosis (fatty liver disease) and collagen deposition.
They observed that these changes correlated with reduced preparative (protective) macrophages. Because transcriptional analysis showed that Gpr18 was increased in aged macrophages relative to young, they investigated its role in aging by generating a conditional knockout mouse wherein only myeloid cells lack GPR18 and through treatment of mice with GPR18's ligand, RvD2.
Together, their studies demonstrated that myeloid-specific GPR18 limited steatosis and collagen accumulation in the liver. Furthermore, adding RvD2 from an external source to activate GPR18 improved liver histopathology. They also found that RvD2 treatment increased bone marrow and blood monocytes, as well as their precursors.
To examine how bone marrow function contributed to liver pathology, they conducted bone marrow transplants in which they reconstituted young mice with the marrow from either young or old animals, with or without RvD2 treatment.
Dr. MacNamara noted, "These studies revealed that donor marrow from aged animals was sufficient to induce collagen accumulation in the liver, demonstrating that aging bone marrow contributes to liver pathology. Importantly, however, this could be improved with RvD2 treatment."
Dr. Fredman commented, "Together, these studies demonstrate that RvD2-GPR18 signaling controls steatosis and fibrosis and provides a mechanistic-based therapy for promoting liver repair in aging."
The investigators were surprised by the specificity of RvD2's actions on the bone marrow and the observation that RvD2, when added ex vivo, acted directly on the bone marrow to induce a specific increase in monocyte/macrophage progenitors.
Dr. MacNamara elaborated, "This was exciting because while much has been studied regarding how specialized proresolving lipid mediators impact macrophage function, much less is known about their impact on blood cell production. As monocytes and macrophages are important for tissue homeostasis throughout the body, in essentially all organs, we believe the ability of RvD2 to increase monocyte production will be relevant to many diseases or aging contexts in which tissue repair is hindered."
Dr. Fredman added, "Perhaps the biggest surprise was that even a very short treatment with RvD2 to older bone marrow in recipient mice had such a profound and durable response. We treated bone marrow transplants for just one week and found that even this transient RvD2 treatment could improve liver pathology four months later. These results suggest that RvD2 may participate in some long-term programming of bone marrow, immune or even liver cells."
The investigators concluded that these studies provide a proof-of-concept that RvD2 can alleviate established liver scarring or fibrosis, for which there is currently no treatment, and that its action may in part, be due to the regulation of bone marrow.
According to Dr. MacNamara, "These studies reveal a potential therapy that may improve pathologies associated with aging by improving the process of blood cell production. The idea that bone marrow production can be modulated to generate cells that provide reparative functions may be broadly relevant in aging."
"Our studies not only highlight the remarkable durability of even transient treatment with RvD2, but they demonstrate the important role of bone marrow and its function in blood cell generation as a key aspect to treating disease. We believe there is tremendous promise of specialized proresolving lipid mediators, like RvD2, as therapies that may improve or augment current treatments."
More information: Hannah Fitzgerald et al, Resolvin D2–G-Protein Coupled Receptor 18 Enhances Bone Marrow Function and Limits Steatosis and Hepatic Collagen Accumulation in Aging, The American Journal of Pathology (2023). DOI: 10.1016/j.ajpath.2023.08.011