This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research finds one of the deleted genes linked to Williams syndrome is responsible for mitochondrial function in brain
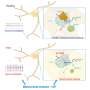
In a first, Tel Aviv University researchers have discovered that the production and regulation of mitochondrial organelles in the brain's nerve cells (neurons) becomes significantly impaired with the deletion of a gene called Gtf2i, one of the 25 genes deleted in Williams syndrome.
This impairment is known to cause functional disability of the nerve cells and may underlie neurodevelopmental pathologies such as Williams syndrome and other conditions associated with the Gtf2i gene.
The discovery emerged from the efforts of a team of researchers spearheaded by Prof. Boaz Barak of the Sagol School of Neuroscience and the School of Psychological Sciences at Tel Aviv University and Ariel Nir-Sade, for whom this groundbreaking research constitutes her doctoral thesis.
"We have 100 billion nerve cells in the brain that are essential for maintaining brain activity," explains Prof. Barak. "In order to do this, these cells require energy. This energy is produced in the mitochondrial organelle; therefore, a problem with the mitochondrial function will lead to a problem with the functioning of the cell."
"Today, we understand that the mitochondria are 'to blame' for a variety of neurological pathologies, from neurodevelopmental disorders such as Angelman syndrome and autism to neurodegenerative diseases like Alzheimer's and Parkinson's—these are all disorders that involve, among other things, abnormal functioning of the mitochondria."
In Prof. Barak's laboratory, the researchers focus on a genetic syndrome called Williams syndrome, which results from the defective expression of about 25 genes. Until now, it was not clear why the nerve cells of those with the syndrome are damaged—that is, what the correlation was between the faulty expression of these genes and the resultant impairment of brain function.
"Williams syndrome is a relatively rare developmental neurogenetic syndrome," explains Nir-Sade. "Individuals with this condition are born with multisystemic deficits from birth, cognitive, motor and behavioral problems, but perhaps their most distinguishing trait is their difficulty in regulating social behavior. That is why it's often called the 'love syndrome'—these individuals tend to exhibit considerable affection and a strong desire for social interaction."
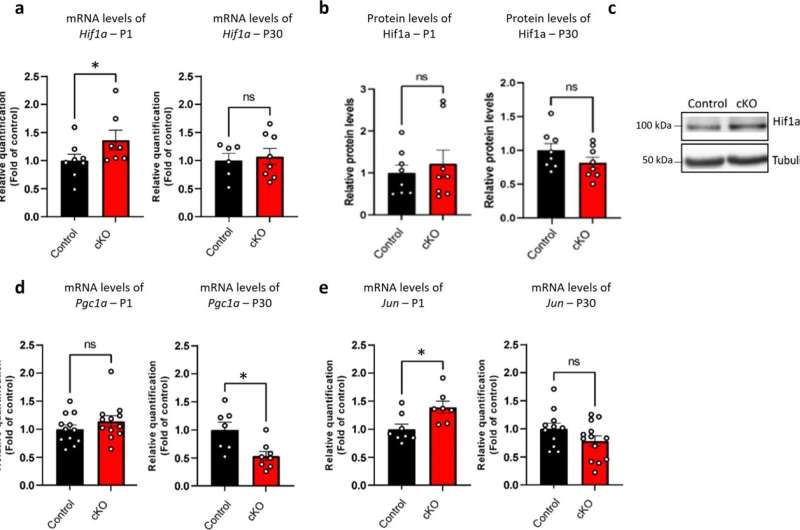
Among the 25 genes that are not properly expressed in individuals with Williams syndrome, Prof. Barak and Nir-Sade's research has focused on the Gtf2i gene. This gene is key for understanding the syndrome, as it encodes a transcription factor—a protein that is responsible for regulating numerous other genes and, as they discovered in their research, for regulating the expression of genes involved in mitochondria.
In their pursuit to comprehend the role of this gene in the brain's nerve cells, the Tel Aviv University researchers employed genetic engineering techniques to compare the mitochondrial structure in nerve cells with and without the Gtf2i gene.
Typically, mitochondria work together in the form of a network, but in the absence of the Gtf2i gene, the process of forming the network is not properly regulated. As a result, the formation of the network is impaired, the mitochondria have difficulty functioning, and abnormal substances accumulate inside the cell.
"In the first stage, we extracted nerve cells from the brains of animal models of Williams syndrome and grew them in culture," says Nir-Sade. "We compared normal nerve cell cultures to those in which the Gtf2i gene had been deleted using genetic engineering. We examined the individual cell and demonstrated how the mitochondrion has difficulty developing and functioning without this gene."
"In the second stage, with the help of Dr. Asaf Marco's laboratory at the Hebrew University of Jerusalem, we wanted to see if the basic mechanism we discovered in animal model cultures would also be valid for human subjects. We examined brain tissue obtained from individuals born with Williams syndrome in which their brains were donated to scientific research after passing away."
"We observed that our findings hold true for human brains as well: In individuals with Williams syndrome, the mitochondria do not develop and function properly, and as a result, toxic material accumulates inside the nerve cell, thereby affecting their efficiency."
"These findings hold clinical importance," adds Prof. Barak. "They improve our understanding of what is required to improve neural function in the brain—for example, improving mitochondrial function or reducing the expression level of the substances that accumulate in the nerve cells of people with Williams syndrome. Biomedical research dedicates substantial effort and resources toward understanding mitochondrial diseases, and significant progress is underway in this area."
"It's plausible that in the future, a drug will be developed to improve mitochondrial function in other conditions, such as Alzheimer's, and based on our research, they will know how to adapt the drug for Williams syndrome as well, with the aim of improving mitochondrial function in this specific context."
The work is published in the journal Communications Biology.
More information: Ariel Nir Sade et al, Neuronal Gtf2i deletion alters mitochondrial and autophagic properties, Communications Biology (2023). DOI: 10.1038/s42003-023-05612-5