This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Evolutionary origin of mysterious immune system molecule in humans revealed
Biological systems can behave as siblings in several ways, including by borrowing something and never giving it back. That appears to be what the human immune system did with a protein that now helps bind and regulate the subunits that make up antibodies, according to a multi-institute research collaboration. They found that, before the immune system evolutionarily co-opted it, the protein originally belonged to gene family responsible for directing cells to move to the right location at the right time to address specific functional needs.
The researchers, including Kazuhiko Kawasaki, associate research professor of anthropology at Penn State, published their findings in the Proceedings of the National Academy of Sciences. According to the team, while this work primarily informs a fundamental understanding of one feature of the immune system and associated genes, it may also help open design pathways future therapeutics, such as personalized immune responses.
"Everything comes from somewhere, and we believe we found the origin of immunoglobin Joining chain (J chain), an important immune molecule," said corresponding author Martin F. Flajnik, department of microbiology and immunology, University of Maryland, who led the study. Flajnik also earned his undergraduate degree in biology from Penn State in 1978 before completing his graduate degrees at the University of Rochester.
The J chain assembles and stabilizes two types of antibodies, called immunoglobin M (IgM) and immunoglobin A (IgA). It specifically regulates the structures of the IgM and IgA molecules, which have several subunits, and is required for their movement across the mucus-producing tissue lining body structures with external exposure, like the intestine, nasal cavity and lungs.
The researchers found that the J chain originated from the CXCL chemokines, a specific family of proteins that regulate the ability of white blood cells to move throughout the body.
"Like immunoglobin itself and human-like adaptive immunity, the J chain emerged in jawed vertebrates, but its origin has remained mysterious since its discovery over 50 years ago," Flajnik said. "This finding was never anticipated. Chemokine-driven locomotion is a vital function of the immune system, but a totally different function as compared to the J chain!"
Evolutionarily, new genes are often generated from genes that reside physically close together on the chromosome, and those genes typically remain clustered together even as they evolve different yet similar functions, but Kawasaki said location isn't the only deciding factor to determine origin.
"The evolutionary relationship of genes can usually be detected when two genes retain similar nucleotide sequences or encoded amino acid sequences," Kawasaki said, referring to the materials comprising an organism's genetic code. "But previous studies could not detect any genes that show sequence similarities to the J chain gene, probably because the J chain gene sequence was quickly changed at its origin."
Flajnik said he had a hunch that the J chain was related to a group of secretory calcium-binding phosphoprotein (SCPP) genes due to their similar charges and levels of proline, an amino acid. He knew Kawasaki was an expert on SCPP genes, so he emailed him to assess the idea.
"He told me that, for various good reasons, the SCPPs and J chain were not related," Flajnik said. "That was sad, as it was my favorite hypothesis."
However, Kawasaki had noticed that genes on the opposite side of the J chain gene, away from the SCPP genes, did appear to be related to the J chain. Those were the CXCL chemokine genes.
"I immediately checked these CXCL chemokine genes and found that, though these genes do not show sequence similarities to the J chain genes, these genes and the J chain gene resemble each other with other various characteristics," Kawasaki said.
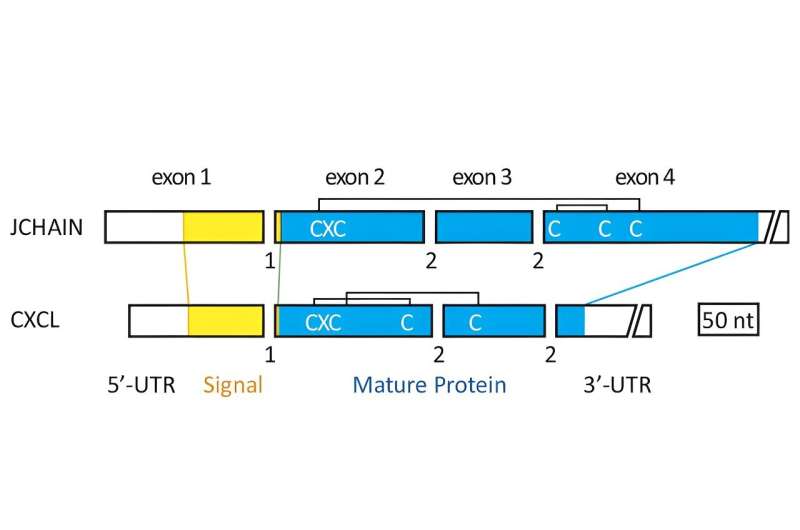
Those characteristics include the same number of exons, which encode the protein, and phases of introns, which act as interrupters to stop or start splicing of the RNA molecules transcribed from the gene. The second exon encodes the same sequence, which is known as the classical tripeptide cysteine-X-cysteine, for both genes. The lengths of three of the exons are also similar.
"No other gene encoding the human secretome, which encompasses all proteins that can be secreted by cells of an organism, shares all three characteristics," Kawasaki said.
The bonds between the cysteine molecules encoded by the second exon in each gene are completely different from one another, though, the researchers said.
"This means that a chemokine can change its structure, to a large extent, and take on a new function," Flajnik said.
Next, the researchers said they plan to investigate if chemokines have taken on other functions, specifically in the immune system. They also want to study if chemokines are pliable in their structure, which could indicate the ability to take on an entirely new secondary structure, adapting in response to different biological needs as required.
"I've been around for a long time, for 44 years in science, but this experience was one of the most incredibly satisfying and lucky," Flajnik said. "I doubt that this similarity would have been uncovered for a long time without the serendipitous interaction between Kazuhiko and me."
More information: Kazuhiko Kawasaki et al, The immunoglobulin J chain is an evolutionarily co-opted chemokine, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2318995121