This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New frontier in glioblastoma theragnosis: Visualizing the tumor microenvironment
Recently, a team of South Korean researchers led by nuclear medicine physician and Professor Yun Mijin from Severance Hospital in collaboration with Director C. Justin Lee of the Center for Cognition and Sociality within the Institute for Basic Science (IBS) made a new discovery that could revolutionize both the diagnosis and treatment of glioblastoma.
The group demonstrated a mechanism where the astrocytes in the brain uptake elevated levels of acetates, which turns them into hazardous reactive astrocytes. The researchers then developed a new imaging technique that takes advantage of this mechanism to directly observe the tumor microenvironment. The study is published in the journal Neuro-Oncology.
Glioblastoma multiforme (GBM) is the most aggressive type of brain cancer with a median overall survival (OS) of approximately 15 months, and it accounts for the majority of primary malignant brain tumors. One of the hallmark features of GBM is the formation of a tumor microenvironment (TME). The TME comprises various cell types, including cancer stem cells and non-tumorous cells, such as astrocytes.
In TME, astrocytes undergo reactive astrogliosis which contributes to the proliferation, invasion, and drug resistance of glioblastoma. In general, glioblastomas are typically treated by surgically excising the tumor through total resection, and magnetic resonance imaging (MRI) is primarily utilized for preoperative localization.
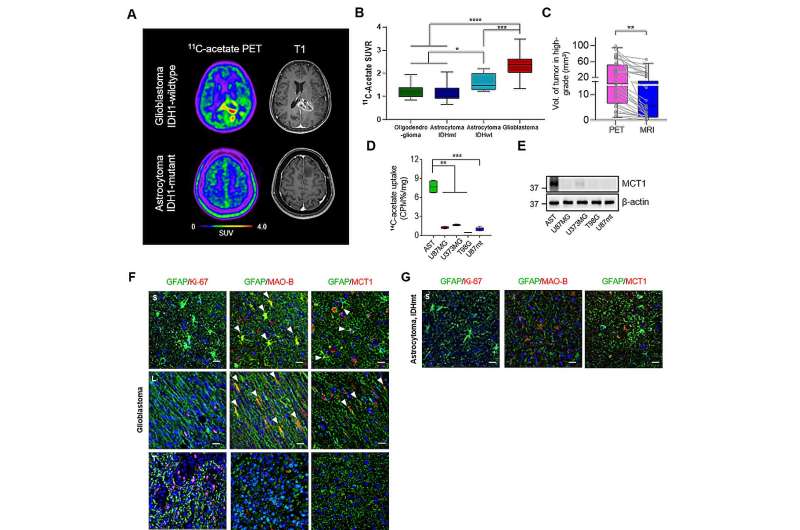
These reactive astrocytes are known to excessively metabolize acetate, which allows them to be easily tracked via radioisotope imaging. To achieve this purpose, Yun and Lee's research team introduced the use of Carbon-11 acetate (11C-acetate) as a probe for Positron Emission Tomography (PET). Earlier this year, they presented a study using 11C-acetate PET imaging to visualize reactive astrocytes in Alzheimer's patients. This time, Yun's team ventured to apply the same approach to glioblastoma.
This approach was found to be particularly useful when tracking glioblastoma compared to other cancers. The 11C-acetate radiotracer is conventionally used to image cells that excessively uptake acetate, such as tumor cells. In the case of glioblastoma, both the reactive astrocytes in the TME uptake acetate as well as the tumor itself, which allows the PET imaging to track and distinguish both components of the cancer (Figure 1A to 1D).
Nuclear medicine physician Kim Dongwoo, one of the first authors of this paper, stated, "PET imaging with 11C-acetate PET can prove to be an effective technique in diagnosing and determining the surgical resection areas of glioblastoma, showcasing significant clinical value."
The research team investigated further to see whether they could take advantage of the acetate metabolism feature for therapeutic purposes as well. Glioma cells are known to monopolize glucose for themselves, which leaves the tumor environment without much glucose, except for the glioma cells. The TME components such as reactive astrocytes are then forced to utilize acetate from the glioma cells for energy.
The astrocytes uptake acetate through a doorway called monocarboxylate transporter 1 (MCT1) (Figure 1E). When astrocytes take in too much acetate, it leads to a condition called reactive astrocytosis (see Figure 1F to 1G). In experiments with the mouse glioblastoma model, inhibiting reactive astrocytosis or blocking the expression of MCT1 was shown to restore acetate uptake in the tumor microenvironment to normal levels (Figure 2A to 2D).
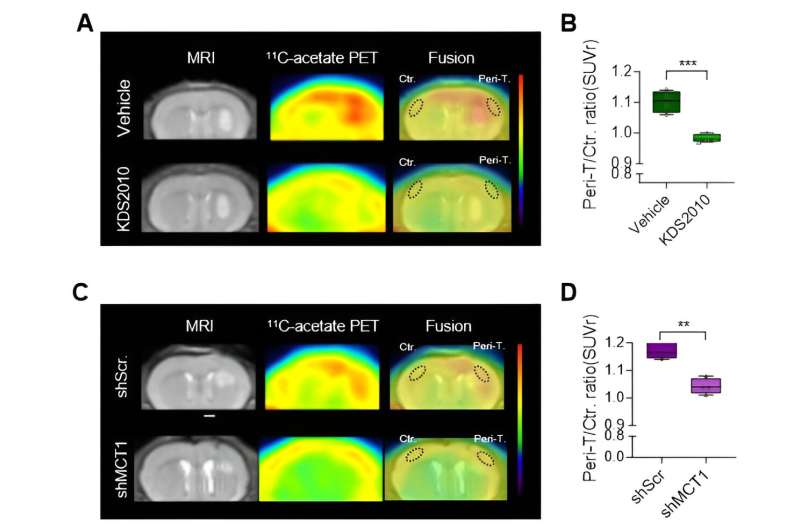
Dr. Yun Mijin commented, "We showed that reactive astrocytes could be a great therapeutic target for glioblastoma," and added, "Combining therapeutics and PET imaging with 11C-acetate as the radiotracer has significant importance in the aspect of 'theragnosis' (therapy + diagnosis)."
In addition, Yun and Lee's team discovered that the prognosis of glioblastoma patients is less favorable when the areas of the TME identified through 11C-acetate PET imaging are larger compared to the tumor areas identified through MRI. This finding underscores the crucial role of the TME in the prognosis of glioblastoma patients.
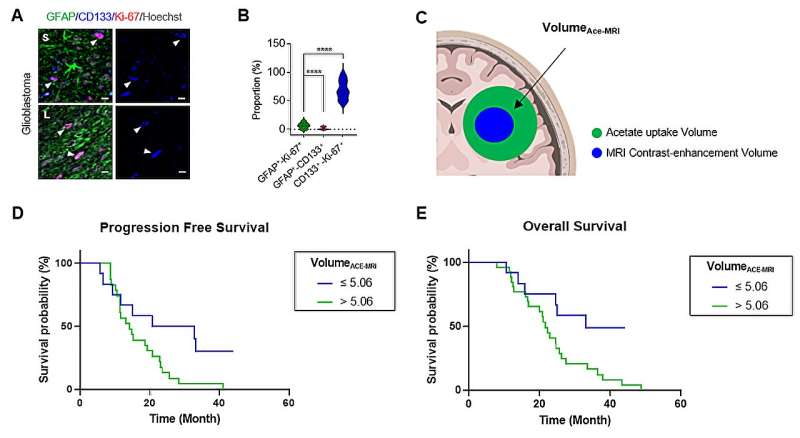
The researchers emphasized the importance of the TME components, consisting of reactive astrocytes and cancer stem cells, in the progression and metastasis of tumors. They highlighted that successfully removing these components through surgical procedures is essential for the successful resection of tumors (Figure 3A to 3E).
Director C. Justin Lee said, "Beyond tumor cells and surrounding reactive astrocytes, there is a need for subsequent macroscopic studies to explore how cancer stem cells, which constitute the TME, and the surrounding neurons influence each other."
More information: Dongwoo Kim et al, Visualizing Cancer-Originating Acetate Uptake Through MCT1 in Reactive Astrocytes in the Glioblastoma Tumor Microenvironment, Neuro-Oncology (2023). DOI: 10.1093/neuonc/noad243