This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Targeting FSP1 regulates iron homeostasis in drug-tolerant persister head and neck cancer cells
A new research paper titled "Targeting of FSP1 regulates iron homeostasis in drug-tolerant persister head and neck cancer cells via lipid-metabolism-driven ferroptosis" has been published in Aging.
Research has demonstrated that some tumor cells can transform into drug-tolerant persisters (DTPs), which serve as a reservoir for the recurrence of the disease. In this new study, researchers Yang-Che Wu, Chin-Sheng Huang, Ming-Shou Hsieh, Chih-Ming Huang, Syahru Agung Setiawan, Chi-Tai Yeh, Kuang-Tai Kuo, and Shao-Cheng Liu from Taipei Medical University-Shuang Ho Hospital, Taipei Medical University, Taitung Mackay Memorial Hospital, Tajen University, National Taitung University, and Taipei City's National Defense Medical Center have investigated lipid-metabolism-driven ferroptosis and its role in drug resistance and DTP generation in head and neck squamous cell carcinoma (HNSCC).
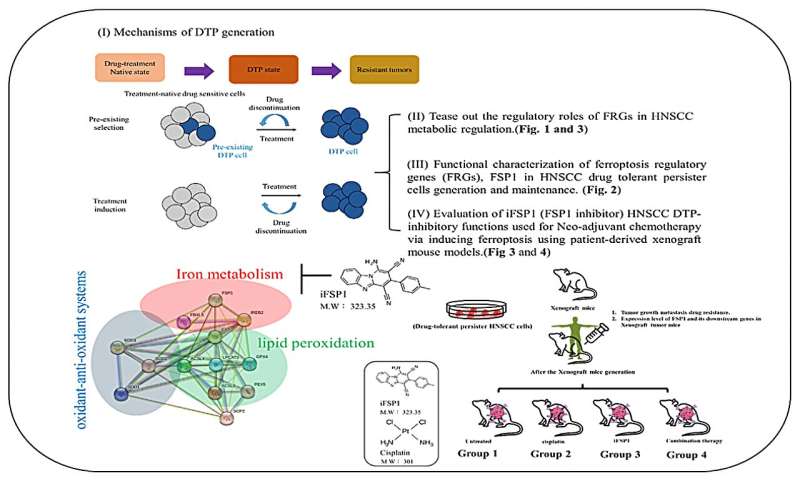
"The regulatory roles of ferroptosis suppressor protein 1 (FSP1) in HNSCC metabolic regulation were investigated," state the researchers.
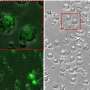
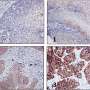
High levels of FSP1 were discovered in the tissues of patients who experienced relapse after cisplatin treatment. RNA sequencing indicated that a series of genes related to lipid metabolism was also highly expressed in tissues from these patients. Consistent results were obtained in primary DTP cells isolated from patients who experienced relapse. The Cancer Genome Atlas database confirmed this finding. This revealed that the activation of drug resistance in cancer cells is influenced by FSP1, intracellular iron homeostasis, and lipid metabolism.
Next, the team generated human oral squamous cell carcinoma DTP cells (HNSCC cell line) to cisplatin and observed higher expression of FSP1 and lipid-metabolism-related targets in vitro. The shFSP1 blockade attenuated HNSCC-DTP cell stemness and downregulated tumor invasion and the metastatic rate. They found that cisplatin induced FSP1/ACSL4 axis expression in HNSC-DTPC cells.
Finally, the researchers evaluated the HNSCC CSC-inhibitory functions of iFSP1 (a metabolic drug and ferroptosis inducer) used for neo-adjuvant chemotherapy; this was achieved by inducing ferroptosis in a patient-derived xenograft mouse model.
"The present findings elucidate the link between iron homeostasis, ferroptosis, and cancer metabolism in HNSCC-DTP generation and acquisition of chemoresistance. The findings may serve as a suitable model for cancer treatment testing and prediction of precision treatment outcomes. In conclusion, this study provides clinically oriented platforms for evaluating metabolism-modulating drugs (FSP1 inhibitors) and new drug candidates of drug resistance and ferroptotic biomarkers," the team concludes.
More information: Yang-Che Wu et al, Targeting of FSP1 regulates iron homeostasis in drug-tolerant persister head and neck cancer cells via lipid-metabolism-driven ferroptosis, Aging (2024). DOI: 10.18632/aging.205409