This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research identifies PLK4 as promising therapeutic target for TP53 mutated acute myeloid leukemia

A research team led by Professor Anskar Leung Yu-hung, from the Department of Medicine, School of Clinical Medicine, LKS Faculty of Medicine, the University of Hong Kong (HKUMed), has identified PLK4 as a novel therapeutic target for acute myeloid leukemia (AML) carrying the TP53 mutation. AML is a deadly disease, for which there is currently a lack of effective treatment options.
The results may provide the mechanistic foundation for setting up clinical trials in this AML subtype with a view to improving patient outcomes. Queen Mary Hospital is one of the treatment sites in which the effect of a PLK4 inhibitor in AML patients will be tested. The study was published in the journal Blood.
AML is a type of blood cancer caused by genetic changes in blood stem cells in bone marrow. Its symptoms include fever, bleeding and infection. Without treatment, AML patients may deteriorate rapidly and die. Conventional treatments include intensive chemotherapy and blood stem cell transplantation. Overall, only 40% of patients can be cured and enjoy long-term survival.
A subtype of AML, carrying a mutation of a tumor-suppressive gene, known as TP53, responds poorly to conventional treatment, resulting in a high mortality rate within a year after diagnosis. At present, there is no specific treatment available for this AML subtype, underscoring the urgent need to develop novel and specific therapies for this disease.
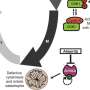
A comprehensive analysis of gene expression and pharmacological vulnerabilities in different AML subtypes identified a gene known as polo-like kinase 4 (PLK4), which is specifically active in TP53 mutated AML. PLK4 is a major regulator of cell division. TP53 mutated AML is resistant to chemotherapy and highly vulnerable to prolonged PLK4 inhibition. PLK4 inhibition also induces DNA damage, cell aging and abnormal cell division.
The team discovered that the combined effects of histone modification and polyploidy activate the cGAS-STING pathway, which triggers the immune system. These findings have been consistently observed in both the laboratory setting and animal models. The combination of the PLK4 inhibitor with a monoclonal antibody against CD47 enhanced macrophage killing capability, synergistically reducing the leukemic burden and resulting in prolonged animal survival.
This is the first study to demonstrate the therapeutic effect of PLK4 inhibition on TP53 mutated AML and the novel therapeutic mechanism pertaining to the activation of the cGAS-STING pathway and the immune system. These observations lay a foundation for evaluating the clinical effects of PLK4 inhibitor in patients with this AML subtype.
In addition to hospitals in the U.S. and Canada, the Hematology Division at Queen Mary Hospital will become a treatment site that is participating in global clinical trials to test the effects of PLK4 inhibitor in AML patients.
More information: Cheuk-Him Man et al, Inhibition of PLK4 remodels histone methylation and activates the immune response via the cGAS-STING pathway in TP53-mutated AML, Blood (2023). DOI: 10.1182/blood.2023019782