This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
RNA sequencing analysis may yield better diagnosis, targeted treatment of pediatric B-acute lymphoblastic leukemia
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer, representing more than 30% of all pediatric cases. A pilot study in The Journal of Molecular Diagnostics confirms the feasibility of implementing an RNA sequencing analysis (RNA-Seq) workflow for clinical diagnosis of molecular subtypes in pediatric B-acute lymphoblastic leukemia (B-ALL).
This promising and cost-efficient global genomic assay for B-ALL may lead to more accurate diagnosis as well as targeted treatment options.
ALL comprises a constellation of diverse molecular subtypes, each with their own individual drug sensitivity pattern, treatment response, and even prognosis. It is important to identify specific subtypes, particularly in the current era of personalized medicine. However, identification requires an array of profiling tools, making the process laborious and expensive.
Co-lead lead investigator Gordana Raca, MD, Ph.D., Department of Pathology and Laboratory Medicine, Children's Hospital Los Angeles, explains, "The goal of the study was to address limitations of current standard-of-care (SOC) testing for pediatric B-ALL, which cannot identify multiple recently discovered subtypes of the disease. We also wanted to take advantage of expression data generated by the enrichment-based RNA-Seq assay clinically validated in our laboratory for detection of oncogenic fusions in cancer to evaluate if additional data analyses could help determine subtype classification in addition to fusion information for our B-ALL cases."
Investigators reviewed archival clinical, morphologic, immunophenotypic, and molecular data as well as residual DNA, RNA, and frozen bone marrow aspirate and/or leukemic peripheral blood samples from previous clinical testing in a group of 76 pediatric patients. Results were analyzed from 61 newly diagnosed patients and 25 patients who had relapsed/refractory B-ALL who underwent cytogenetic and molecular characterization as part of their standard clinical care at the Center for Personalized Medicine at Children's Hospital Los Angeles between March 2016 and September 2020. The team hypothesized that RNA-Seq, which has been used in the discovery of novel molecular subtypes of B-ALL, could also be a clinically useful tool for diagnostic classification of B-ALL cases.
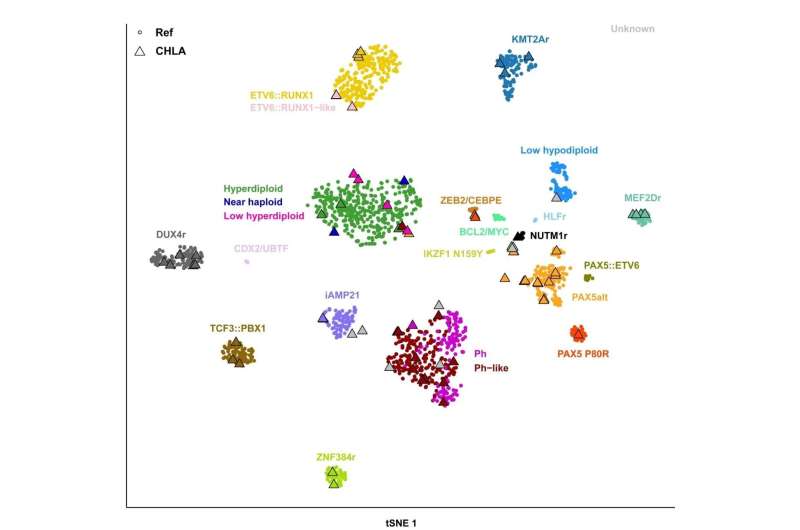
To test this hypothesis, investigators analyzed RNA-Seq data in 28 cases of B-ALL with known subtype and 48 with undetermined subtype following clinical karyotype analysis, fluorescence in situ hybridization, chromosomal microarray, and next-generation sequencing DNA and RNA fusion panel testing (OncoKids). RNA-Seq analysis accurately identified the subtypes in all 28 known cases and determined the genetic subtype in 38 of the 48 previously unknown cases (79%). RNA-Seq analysis was also able to detect oncogenic fusions, large copy number abnormalities, oncogenic hot-spot sequence variants, and intragenic IKZF1 deletions.
Dr. Raca adds, "RNA-Seq analysis enables accurate determination of the genetic subtype for an increased proportion of pediatric B-ALL cases, which will allow more accurate risk stratification and optimization of patient management. Accurate subtype determination at diagnosis will also grow our knowledge about morphologic, immunophenotypic, and clinical characteristics of novel B-ALL subtypes. We were surprised by a relatively high frequency of some novel B-ALL subtypes (like PAX5Alt and DUX4) in our patient cohort, which remain undiagnosed by SOC testing. The study also resulted in discovery of several previously unreported fusions."
Dr. Raca says, "The study showed that expression-profile–based classification, which to date has been mostly used in research, can also be a very powerful clinical assay for pediatric B-ALL. As a single test, it allows subtype determination in a higher proportion of cases than comprehensive multi-modal SOC testing performed at our institution. Therefore, it has the potential to increase both diagnostic yield and efficiency of B-ALL testing."
In an accompanying editorial, Shawn H.R. Lee, MD, Khoo Teck Puat–National University Children's Medical Institute, National University Health System, Singapore, and Department of Pediatrics, Yong Loo Lin School of Medicine, National University of Singapore, concurs that RNA-seq is arguably one of the most—or even potentially the most—powerful single tool for somatic profiling in pediatric B-ALL. However, he also notes that it still remains contentious whether RNA-Seq as the sole means of classification is recommended for all cases given some inherent limitations, especially in locations where analytical pipelines may not be as robust.
Dr. Lee comments, "Overall, RNA-seq as a single somatic genomic ALL diagnostic platform holds immense promise, although still with associated gaps. In some cases, validation of positive cases by an alternative method (such as oncogene fusion test and DNA sequencing) may still be arguably a sounder approach. The test needs further refining to comprehensively obtain mutation information beyond that of molecular subtyping to make it globally accessible. As we move further into the era of precision medicine, the information provided by this test holds excellent promise in guiding relevant and risk-stratified treatment in this childhood cancer."
Co-Lead investigator Zhaohui Gu, Ph.D., Department of Computational and Quantitative Medicine & Systems Biology, Beckman Research Institute of City of Hope, Duarte, says, "The study showed that B-ALL cases with known subtypes by SOC testing were fully concordant with the RNA-Seq–based classification. In addition, RNA-Seq analysis allowed us to successfully classify a large proportion of cases that remained unknown upon comprehensive SOC testing. RNA-Seq–based classification was relatively easy to implement in a medium-size academic laboratory using data from a clinically validated fusion assay."
More information: Zunsong Hu et al, Transcriptome Sequencing Allows Comprehensive Genomic Characterization of Pediatric B-Acute Lymphoblastic Leukemia in an Academic Clinical Laboratory, The Journal of Molecular Diagnostics (2023). DOI: 10.1016/j.jmoldx.2023.09.013
Shawn H.R. Lee, Toward a Comprehensive One-Stop Shop for Somatic Genomic Profiling in Childhood Acute Lymphoblastic Leukemia, The Journal of Molecular Diagnostics (2023). DOI: 10.1016/j.jmoldx.2023.11.001