This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Towards a better understanding of endothelial cell transformation in cancer progression
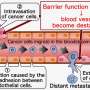
Endothelial-mesenchymal transition (EndoMT, also termed as EndMT), a biological process resulting in the formation of mesenchymal (or lineage-committed) phenotypes from endothelial cells (lining blood vessels), plays a crucial role in tumor progression. Despite the important role of EndoMT, the underlying mechanism and characteristics of cells in intermediate/partial EndoMT remain largely unexplored. Now, researchers from Japan have developed a system to study these EndoMT stages.
In a study published recently in Cancer Science, researchers from Tokyo Medical and Dental University (TMDU), Japan, report a system that can detect the intermediate and full stages of EndoMT.
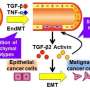
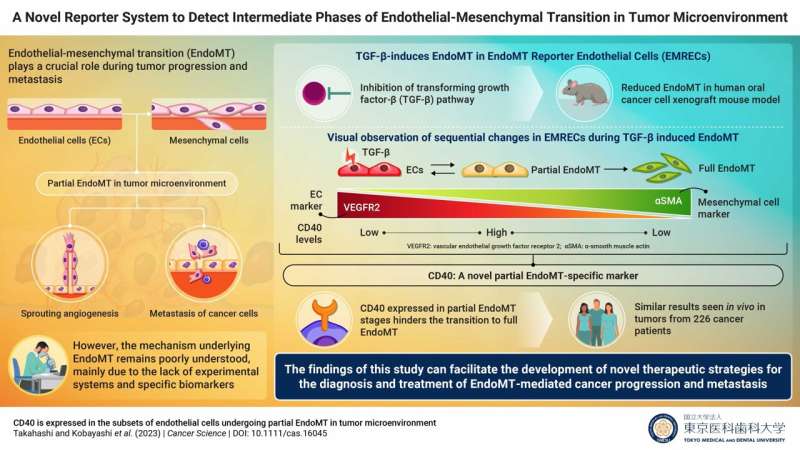
Named "EndoMT Reporter Endothelial Cells (EMRECs)," this novel reporter cell experimental system enables the visualization of the sequential changes that occur during EndoMT induced by transforming growth factor-β (TGF-β). TGF-β is a cytokine found abundantly in the tumor microenvironment (TME) and is known to play a key role in EndoMT.
"In the present study, we established EMRECs to be able to visualize EndoMT transition in real-time and identified specific genes expressed in partial EndoMT during diseases like cancer," explains the lead author of the study, Dr. Kazuki Takahashi.
To visualize the intermediate stages of EndoMT, the researchers developed an EndoMT-reporter transgenic mouse that expressed a red fluorescent protein under the control of an endothelial cell-specific marker and a green fluorescent protein under the control of α-smooth muscle actin (αSMA), a mesenchymal cell marker. Cells of endothelial lineage derived from this transgenic mouse would emit red fluorescence, providing insight into the endothelial lineage.
EMRECs were established using immortalized endothelial cells from the transgenic mouse liver. Once EMRECs acquired mesenchymal cell characteristics, they would emit green fluorescence due to the expression of αSMA—a mesenchymal cell marker—resulting in a gradient change from orange to yellow, as cells express both the endothelial-lineage and mesenchymal markers.
In addition, simultaneous detection of the expression of the endothelial cell marker 'vascular endothelial growth factor receptor 2 (VEGFR2)' in EMRECs can provide a more detailed understanding of the EndoMT sequential stages: the full EndoMT stage would no longer express VEGFR2, but the partial EndoMT stages with both endothelial and mesenchymal properties would still express VEGFR2.
Quantitative RT-PCR (qRT-PCR) and immunohistochemical analyses of EMRECs revealed that treatment with TGF-β increased the expression of αSMA and lowered the expression of VEGFR2, indicating that EMRECs underwent TGF-β-induced EndoMT. Also, inhibition of TGF-β signaling in a human oral cancer xenograft mouse model reduced the ratio of cells expressing both endothelial and mesenchymal markers (indicating EndoMT) in tumors, suggesting that TGF-β inhibition suppresses EndoMT.
To observe the sequential changes of gene expression during EndoMT, the researchers cultured EMRECs with and without TGF-β treatment and used cell sorting assays to specifically pick up cells in stages of partial EndoMT, along with cells that underwent full EndoMT.
Furthermore, by performing RNA sequencing analysis and comparing the cell population of each EndoMT stage, they identified CD40, a surface receptor involved in inflammation, which could serve as a potential partial EndoMT marker, highlighting its crucial role in studying the EndoMT mechanism.
Senior author Dr. Miho Kobayashi says, "Interestingly and for the first time, our results have revealed CD40 as a potential partial EndoMT-specific marker. This is because high CD40 levels were observed in partial EndoMT cell population, but low levels were observed in endothelial and full EndoMT cell populations. We were further intrigued that CD40 suppressed the transition from partial to full EndoMT."
The team also validated tumor tissue samples from 226 patients across 10 solid cancer types by single-cell RNA sequencing analysis and found that the EndoMT pathway results in the formation of cancer-associated fibroblasts (CAFs) through CD40-positive partial EndoMT. CAFs are known to contribute to cancer progression and metastasis.
Senior author Dr. Tetsuro Watabe says, "The EMRECs established in our study can be used for multiple purposes, such as for evaluating the dynamics and distribution of EndoMT in pathological conditions, studying the efficacy of therapeutic agents by live imaging, and screening novel therapeutic drugs targeting each EndoMT stage."
On the whole, these findings improve our understanding of the mechanisms underlying EndoMT, paving the way for novel therapeutic strategies that target EndoMT-mediated cancer progression and metastases.
More information: Kazuki Takahashi et al, CD40 is expressed in the subsets of endothelial cells undergoing partial endothelial–mesenchymal transition in tumor microenvironment, Cancer Science (2023). DOI: 10.1111/cas.16045