This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
FDA panel addresses accuracy issues with pulse oximeters and skin tone

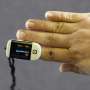
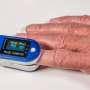
On Friday, a U.S. Food and Drug Administration advisory panel addressed the ongoing issue of less accurate readings from pulse oximeters when used by individuals with darker skin.
During its daylong meeting, the FDA Anesthesiology and Respiratory Therapy Devices Panel reviewed ways to better evaluate the accuracy of pulse oximeters in patients with darker skin. Although there is more work to be done when it comes to making pulse oximeters more accurate, panel member Jeffrey Feldman, M.D., said the benefits of these devices still outweigh their limitations.
"This technology has and continues to save lives on a daily basis in this country. … It needs to be improved. We need to look at health disparities, and we need to do better," he said after the meeting, CNN reported. "But we also need to recognize how valuable this technology is for patients every day, at home, and in the hospital."
Precisely because the general public can use these devices at home to check their oxygen levels, the panel homed in on how to ensure the accuracy of pulse oximeters for all skin tones before they reach drugstore shelves. So, the panel focused on the structure of company trials testing the products.
Back in 2013, the FDA issued premarket guidance for developers of pulse oximeters, recommending that they have "a range of skin pigmentation" represented in their clinical studies of the devices, including at least two "darkly pigmented subjects or 15 percent of the study group," whichever is larger.
Now, the FDA is weighing proposals to update these clinical trials to include more diverse groups of people, with at least 24 participants spanning the entire range of skin tones on the 10-shade Monk Skin Tone scale.
"There's no question that more diversity needs to be a part of whatever new requirements that they would issue," Feldman said. "The prior requirements in 2013 were small numbers and really not very diverse—the only requirement was for up to two patients of color—and so that I think has proven to be inadequate to predict real-world performance."
More information: CNN article.
Copyright © 2024 HealthDay. All rights reserved.