This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Interferon-gamma drives brain pathology in a mouse model of multiple system atrophy
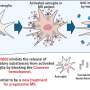
Multiple system atrophy is a rare and fatal neurodegenerative disease, where insoluble inclusions of the protein alpha-synuclein appear in oligodendrocyte cells of the brain. The resulting pathogenesis features neuroinflammation, demyelination and neurodegeneration. Oligodendrocytes produce myelin, an insulating sheath on the axons of nerves.
In 2020, Ashley Harms, Ph.D., and University of Alabama at Birmingham colleagues published an Acta Neuropathologica study that used a mouse model to show that the alpha-synuclein pathology from overexpression of alpha-synuclein in oligodendrocytes induced changes that included infiltration of CD4+ and CD8+ T cells into the brain, as is seen in human post-mortem brains.
The UAB researchers also showed that mice that were genetically deficient in CD4+ T cells had attenuated infiltration of peripheral immune cells and attenuated demyelination in the mouse model. In mice with an intact immune system, alpha-synuclein overexpression in the mouse model resulted in increased numbers of CD4+ T-cells that were also positive for the transcription factor T-bet, along with significant production of the proinflammatory cytokine interferon-gamma, or IFNγ.
Now in a study published in Acta Neuropathologica Communications, Harms and colleagues used the mouse model and genetic and pharmacological approaches to show that IFNγ is produced primarily by infiltrating CD4+ T-cells and that IFNγ mediates the mechanisms that drive multiple system atrophy.
"These results indicate that IFNγ represents a potential future disease-modifying therapeutic target in multiple system atrophy," said Harms, an associate professor in the UAB Department of Neurology. "Future studies are needed to determine the timing and duration of treatment, but these results are promising."
Multiple system atrophy currently has no known disease-modifying therapy.
The mouse model uses an engineered virus that produces overexpression of human alpha-synuclein in oligodendrocytes.
Using mice in which the required transcription factor for IFNγ in Th1 helper T cells, Tbet, has been deleted, the UAB researchers showed that absence of Tbet in the mouse model of multiple system atrophy resulted in attenuated neuroinflammation, demyelination and neurodegeneration.
However, it was still not clear that IFNγ was the driver of that pathology, because Tbet mediates other pathways besides IFNγ.
To specifically determine the role of IFNγ in the mouse model, the researchers gave the mice IFNγ-neutralizing antibody treatment both before and during overexpression of alpha-synuclein. They found that the antibody treatment attenuated neuroinflammation and the entry of CD4+ and CD8+ T cells into the brain, and it reduced demyelination.
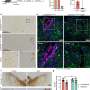
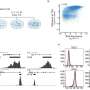
A clever genetic trick—a Thy1.1 reporter mouse—was used to show that most of the IFNγ in the mouse model of multiple system atrophy is produced by CD4+ T cells, rather than other resident or infiltrating immune cells. In this reporter mouse, the gene for Thy1.1 is inserted into the promoter of the IFNγ gene, so that Thy1.1 is co-expressed in any cell that produces IFNγ. Thy1.1 is a cell surface protein, which means that IFN-producing cells can be identified by the presence of Thy1.1.
After alpha-synuclein was overexpressed in the reporter mouse, the researchers removed brain tissue and used immunohistochemistry to identify immune populations known to produce IFNγ—including CD4+ T cells, CD8+ T cells, natural killer cells, astrocytes and microglial cells. They found that the CD4+ T cells expressed the overwhelming majority of Thy1.1 on their cell surface in response to the overexpression of alpha-synuclein.
"These data suggest that the CD4+ T cell effector subtype, Th1 cells, are facilitating the disease process via production of IFNγ," Harms said. "Together our results show that other immune cell types like CD8+ T cells, B cells and natural killer cells do not significantly express IFNγ following alpha-synuclein overexpression in oligodendrocytes; but CD4+ T cells drive multiple system atrophy pathology via IFNγ expression."
More information: Nicole J. Corbin-Stein et al, IFNγ drives neuroinflammation, demyelination, and neurodegeneration in a mouse model of multiple system atrophy, Acta Neuropathologica Communications (2024). DOI: 10.1186/s40478-023-01710-x