This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Parkinson's disease: Essential role in neuroinflammation found for a subset of brain macrophages
In Parkinson's disease, growing evidence targets neuroinflammation as essential for brain pathogenesis. But which group of immune cells that reside in the brain direct this inflammatory response?
In a study published in Nature Communications, Ashley Harms, Ph.D., and colleagues at the University of Alabama at Birmingham used a mouse model of Parkinson's disease to show that border-associated macrophages—not microglia—mediate the neuroinflammatory response in the brain. Neuroinflammation has previously been shown to exacerbate neurodegradation in the mouse model of Parkinson's.
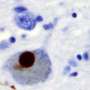
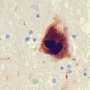
In humans, abnormal accumulation of the protein alpha-synuclein results in the loss of brain neurons that produce the neurotransmitter dopamine. The mouse model—where human alpha-synuclein is overproduced in dopaminergic neurons—replicates many of the inflammatory features seen in human disease.
Microglia and border-associated macrophages, or BAMs, are two specific subsets of macrophage immune cells that reside in the brain. They are also known as central nervous system-resident macrophages, or CRMs. Macrophages are a type of white blood cell that engulf and digest "foreign" pathogens like cancer cells and bacteria. They then play a key role to stimulate the adaptive immune response for large-scale defense by presenting pieces of protein from the pathogen, known as antigens, on the surface of the macrophage. The T cells that are essential to the escalation of the immune response become activated when they interact with those presented antigens. The antigens are presented on the macrophage by a carrier protein called MHCII.
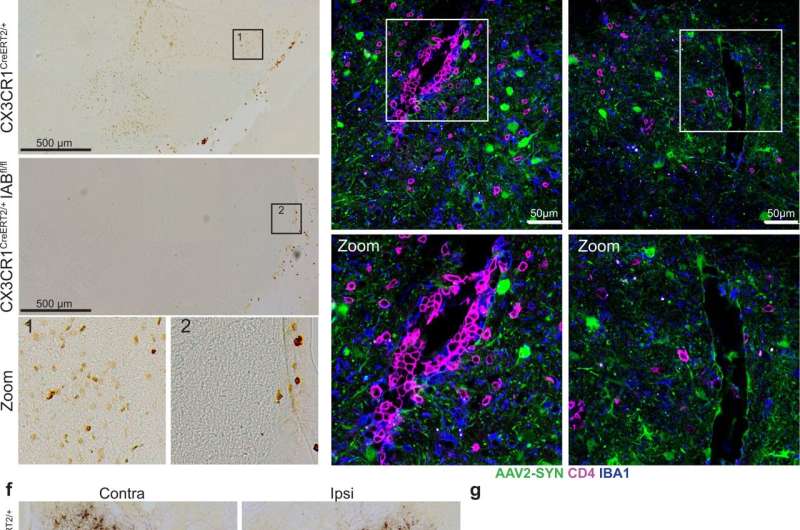
Previous studies had shown that MHCII has a pivotal role in Parkinson's neuroinflammation and neurodegradation. However, identification of the specific cells presenting the antigen remained elusive. The UAB researchers showed that deleting MHCII from CRMs—which includes both microglia and BAMs—was neuroprotective against dopaminergic cell loss in the mouse model, meaning that antigen presentation specifically by CRMs is essential for the inflammation and subsequent neurodegradation induced by alpha-synuclein.
However, Harms and colleagues were surprised to find that deletion of MHCII specifically in microglia caused no reduction in neuroinflammation. Thus, they reasoned that some other non-microglial subset of CRMs must be presenting the alpha-synuclein antigen to CD4+ T cells, thus producing neuroinflammation and the infiltration of peripheral immune cells into the brain from outside the central nervous system.
In contrast to the microglia experiment, they found that specific depletion of BAMs reduced multiple measures of neuroinflammation. "Together, these findings indicate that BAMs, not microglia, are required for peripheral immune cell recruitment into the brain parenchyma and antigen re-stimulation, a process responsible for alpha-synuclein-induced neurodegeneration," said Harms, an associate professor in the UAB Department of Neurology.
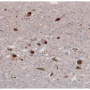
The UAB researchers also showed that alpha-synuclein overexpression prompted CRMs to change into disease-associated microglia and disease-activated BAMs, as identified by specific patterns of gene activation for functions like cytokine signaling and phagocytosis. This activation is similar to activated CRMs that previously have been identified in Alzheimer's disease and amyotrophic lateral sclerosis.
Besides a unique damage-associated activation state, alpha-synuclein expression created an expansion in the numbers of BAMs.
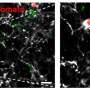
Finally, the researchers tried to correlate their mouse findings to human Parkinson's disease by comparing post-mortem Parkinson's disease mid-brain samples with control mid-brain samples. Intriguingly, they found that Parkinson's disease patients had a significantly higher percentage of the T cell population, both CD4+ and CD8+ T cells, adjacent to BAMs as compared to healthy controls.
"This mirrors what we see in our mouse model of Parkinson's disease, indicating a disease-associated interaction similar to that observed in mice," Harms said.
"These findings change our classical understanding of neuroinflammatory mechanisms in neurodegenerative disease, as they implicate unique and non-redundant functions for BAMs in their role of immune cell recruitment, class II antigen presentation and T cell infiltration," Harms added. "The results point to a critical role for BAMs in neuroinflammatory responses and highlight the need for disease-modifying therapeutic targeting of BAMs in neurodegenerative disease."
BAMs are called "border-associated" because they are found near blood vessels of the central nervous system's borders.
More information: A. M. Schonhoff et al, Border-associated macrophages mediate the neuroinflammatory response in an alpha-synuclein model of Parkinson disease, Nature Communications (2023). DOI: 10.1038/s41467-023-39060-w