February 23, 2024 dialog
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
written by researcher(s)
proofread
Intravesical gemcitabine/docetaxel as an alternative therapy for patients with non-muscle-invasive bladder cancer
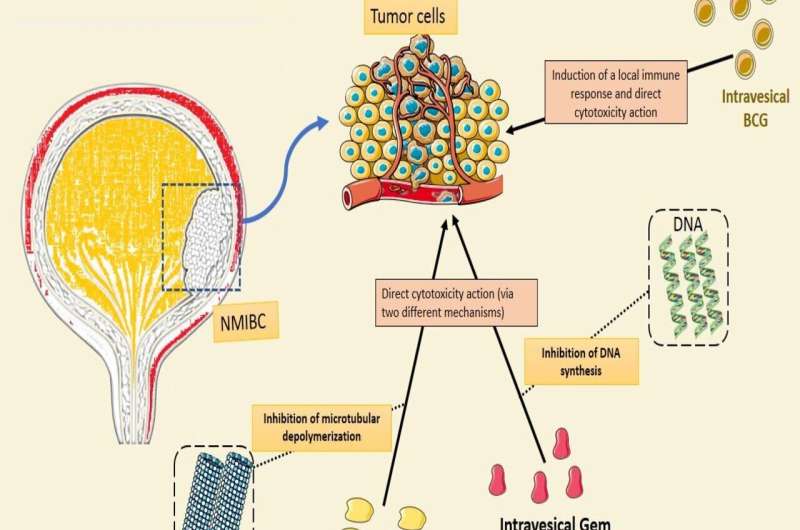
Bladder cancer is one of the more common cancers worldwide. It is considered a major health care problem with a high financial burden. Of these cases, 75% are non-muscle-invasive, which characterizes dangerous diseases with a high risk of recurrence (up to 70% within five years of diagnosis) and progression (up to 40% within five years of diagnosis).
Treatment relies on a risk stratification strategy where risk groups are identified based on tumor characteristics and recurrence history: low risk, intermediate risk and high risk.
The treatment guidelines for non-muscle-invasive bladder cancer (NMIBC) consist of endoscopic resection of the tumor, then administration of drugs into the bladder (intravesical therapy), typically with weekly instillation for six weeks (induction therapy) followed by additional instillations (maintenance therapy) every three or six months or monthly for one to three years.
Intravesical bacillus Calmette-Guerin (tuberculosis vaccine) has been used as adjuvant therapy by urologists since 1976. Because of its reliable reduction in both recurrence and progression, it has become the standard of care for the treatment of HR NMIBC.
Unfortunately, despite its good clinical activity, BCG fails approximately 40% of patients in two years, leading to an ever-present need to develop rescue agents to avoid the need for bladder removal (cystectomy).
Since 2012, when contamination occurred at one of the two major suppliers of BCG resulting in its permanent closure, BCG has been in a worldwide shortage situation. While multiple trials were launched to investigate the use of alternative therapies such as intravesical chemotherapy, immunotherapy and gene therapy, both as a substitute for BCG as well as rescue treatment after BCG failure, none provided the same level of efficacy, safety and affordability of BCG, until recently.
One of the most promising new strategies for NMIBC is the combination of intravesical chemotherapy drugs used as sequential therapy, one drug after the other, with one-hour bladder dwell time for each drug. Initial results with gemcitabine (Gem) and mitomycin C (MMC) appeared promising but shortages in MMC led to the need to substitute docetaxel (Doce) for MMC to create Gem/Doce.
Both were developed by Dr. Michael O'Donnell, professor and director of urologic oncology, at the University of Iowa (UI) in the U.S. Results for Gem/Doce were first reported in 2015 and since then it has been reported in more than 500 patients by multiple institutions.
To summarize the recent data accumulating on combination intravesical sequential chemotherapy for NMIBC, we performed a systematic review that has been published in Expert Opinion on Pharmacotherapy. We included data from 15 trials that were relevant to our aim that included data on Gem/MMC or Gem/Doce.
However, while reports of Gem/MMC were limited to failed BCG patients, with the evolving BCG shortage, Gem/Doce found a use for not only failed BCG patients but as a substitute for patients never previously receiving BCG (BCG naïve).
Efficacy in terms of cancer recurrence was expressed in terms of 24-month, recurrence-free survival. In the BCG-naive group, the 24mRFS for Gem/Doce was ~77% for both HR (all high-grade) and IR (almost all low-grade) diseases. In the HR NMIBC BCG-failure setting, the overall 24mRFS for Gem/Doce was 42%.
Interestingly, Gem/MMC performed similarly well for BCG failures. In terms of side effects, the documented toxicity of Gem/Doce was found to be generally mild in most studies with roughly half of patients reporting no side effects. Grade III (serious) or higher adverse events were rare (<1%). The results for Gem/MMC were also generally mild with rare Grade III toxicity.
In stark contrast, BCG therapy is associated with local side effects in ~60% of cases and systemic side effects in up to 35% of cases, with more than 20% being grade III. Furthermore, roughly 10% of patients drop off BCG treatment compared to ~3% for Gem/Doce.
In simple terms, intravesical Gem/Doce was shown to be an effective and safe treatment for NMIBC if used as first-line therapy in place of BCG and as rescue therapy after BCG failure. Gem/MMC fared near equally well for BCG failure patients but these results were based on less than 100 patients treated.
To define the granular details of where Gem/Doce worked best among HR BCG-failed patients, the 24mRFS was found to be the highest for papillary-only tumors and those that had relapsed after prior successful BCG. BCG-refractory cases (the most severe type of BCG failure) and those with carcinoma in situ (CIS)—an aggressive surface-spreading form of bladder cancer had 24mRFS rates ~10% lower.
However, for these CIS cases, the 24mRFS for Gem/Doce was found to be two- to three-fold better than that of any single intravesical chemotherapy treatment for BCG-unresponsive CIS cases (e.g., Gem, MMC), as well as the three FDA-approved medications (Valrubicin, pembrolizumab, and nadoferagene firadenovec).
A limitation of our analysis to date is that the evidence supporting the efficacy of Gem/MMC and Gem/Doce emerges from single or multi-institutional retrospective (real-world) studies. However, prospective studies are underway including a phase III randomized trial directly comparing BCG versus Gem/Doce in the management of HR BCG-naïve NMIBC (the BRIDGE trial.)
One added notable feature of Gem/Doce is its cost effectiveness. The mean cost per patient at two years is estimated at approximately $12,363 for BCG therapy vs. $7,090 for Gem/Doce (almost saving half of the treatment cost). Newer immunotherapy (such as pembrolizumab) and gene therapy (Nadofaragene firadenovec) are much more expensive despite being less effective.
In a recent model analysis, Nadofaragene firadenovec had a total cost of $346,000, while pembrolizumab had a total cost of $286,000 when used to treat BCG unresponsive CIS disease. The Gem/Doce affordability by virtue of using cheap, widely available, generic drugs should be particularly attractive to health practices at home and abroad that have limited financial resources.
The use of intravesical sequential doublet chemotherapy, like Gem/Doce, is changing the paradigm for treating NMIBC, offering new hope for patients unable to get BCG and for those in whom BCG has already failed them.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
Dr. Michael O'Donnell, MD, is the Richard D. Williams Chaired Professor and Director of Urologic Oncology at the University of Iowa Carver College of Medicine and a member of the Holden Comprehensive Cancer Center. Dr. O'Donnell has pioneered new treatments in non-muscle invasive bladder cancer including combination intravesical immunotherapy and sequential intravesical chemotherapy. He has authored more than 200 peer-reviewed manuscripts, 20 book chapters, and hundreds of scientific abstracts. Currently, Dr. O'Donnell is working with the International Bladder Cancer Group.
Dr. Mohamad Abou Chakra, MD, is a graduated urologist from the Lebanese University and Sorbonne University. He is currently a clinical research fellow in urologic oncology at the University of Iowa. His clinical and research focus is on understanding and treating patients with urothelial carcinoma. He serves on the editorial board of several high-impact journals and has written more than 75 peer-reviewed articles.
More information: Mohamad Abou Chakra et al, Combination intravesical chemotherapy for non-muscle invasive bladder cancer (NMIBC) as first-line or rescue therapy: where do we stand now? Expert Opinion on Pharmacotherapy (2024). DOI: 10.1080/14656566.2024.2310073