This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists identify new 'regulatory' function of learning and memory gene common to all mammalian brain cells
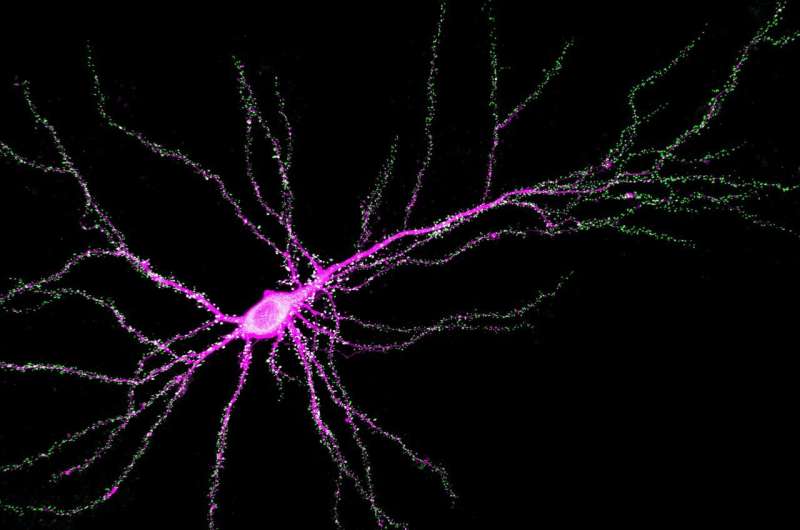
Johns Hopkins Medicine neuroscientists say they have found a new function for the SYNGAP1 gene, a DNA sequence that controls memory and learning in mammals, including mice and humans.
The finding, published in Science, may affect the development of therapies designed for children with SYNGAP1 mutations, who have a range of neurodevelopmental disorders marked by intellectual disability, autistic-like behaviors, and epilepsy.
In general, SYNGAP1, as well as other genes, control learning and memory by making proteins that regulate the strength of synapses—the connections between brain cells.
Previously, the researchers say, the SYNGAP1 gene was thought to work exclusively by encoding a protein that behaves like an enzyme, regulating chemical reactions that lead to changes in the strength of synapses.
Now, the scientists say, their experiments in mice show that protein encoded by the gene may also function more like a so-called scaffolding protein that regulates synaptic plasticity, or how synapses get stronger or weaker over time, independent of its enzyme activity. The SynGAP protein appears to act as a traffic manager, they say, directing where and what brain proteins are at synapses.
With his team, Richard Huganir, Ph.D., Bloomberg Distinguished Professor of Neuroscience and Psychological and Brain Sciences and director of the Solomon H. Snyder Department of Neuroscience at the Johns Hopkins University School of Medicine, first isolated the SYNGAP1 gene in 1998.
SynGAP proteins are very abundant at the synapse, says Huganir, and it has long been thought that SynGAP's main role was to spark enzymatic chemical reactions that regulate synapse strength.
But, working with the SynGAP protein, Huganir and others had begun to see that SynGAP proteins have a strange property when they interact with the major synaptic scaffolding protein, PSD-95. They morph into liquid droplets.
"For an enzymatic protein, that structural transformation is unusual," says Huganir.
To tease out and understand the purpose of SynGAP's peculiar liquid transformation, Huganir, neuroscience instructor Yoichi Araki and Huganir's research team at Johns Hopkins designed experiments in neurons in which they inserted mutations in the so-called GAP domain of the SYNGAP1 gene that would remove the enzymatic function of SynGAP without affecting its structure.
The Johns Hopkins team found that, even without the enzymatic activity, the synapse worked normally, suggesting that the structural property alone is very important for SynGAP function.
The research team next did the same type of genetic engineering in mice to remove the enzymatic function of SynGAP, and found similar results: Synapses behaved normally, with no problems in synaptic plasticity, and the mice had no difficulty in learning and memory behaviors. The research team says this indicates that SynGAP's structural property was sufficient for normal cognitive behavior.
To understand how SynGAP's structure regulates synapses, the scientists analyzed synapses more closely to find that SynGAP protein competed with the binding of AMPA receptor/TARP complexes, a bundle of neurotransmitter proteins that strengthens synapses, and the PSD-95 scaffolding protein.
The experiments suggest that, at rest, SynGAP tightly binds to PSD-95, not allowing it to bind to any other proteins in the synapse. However, during synaptic plasticity, learning and memory, SynGAP protein disconnected from PSD-95, left the synapse and allowed neurotransmitter receptor complexes to bind to PSD-95. This made the synapse stronger and increased transmission between brain cells.
"This sequence happens without the catalytic activity typical of SynGAP," says Huganir. Rather, SynGAP corrals PSD-95 when bound to it, but when SynGAP leaves this synapse, PSD-95 is open to bind to AMPA receptor/TARP complexes.
In children with SynGAP mutations, about half the number of SynGAP proteins are in the synapse. With fewer SynGAP proteins, PSD-95 may bind more with the AMPA receptor/TARP complexes, changing neuronal connections and creating the increased brain cell activity characteristic of epileptic seizures common among children with SynGAP mutations.
Huganir says that both functions of SynGAP—enzymatic and the "traffic management" action of a scaffolding protein—may now be important in finding treatments for SynGAP-related neurodevelopmental disorders. Their research also suggests that targeting just one function of SynGAP alone may not be enough to have a significant impact.
More information: Yoichi Araki et al, SynGAP regulates synaptic plasticity and cognition independently of its catalytic activity, Science (2024). DOI: 10.1126/science.adk1291. www.science.org/doi/10.1126/science.adk1291