This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Shifting focus: Investigators describe changes to pancreatic β cells at onset of type 1 diabetes
About eight million people live with type 1 diabetes (T1D) worldwide, a chronic autoimmune condition in which the body attacks and destroys its own insulin-producing β-cells (pronounced "beta") in the pancreas, leading to a lack of insulin and inability to regulate blood sugar. It's not known why the body suddenly perceives its own β-cells as the enemy; some lines of evidence suggest environmental factors such as viral infections may trigger the onset of T1D, others suggest genetics may also play some role.
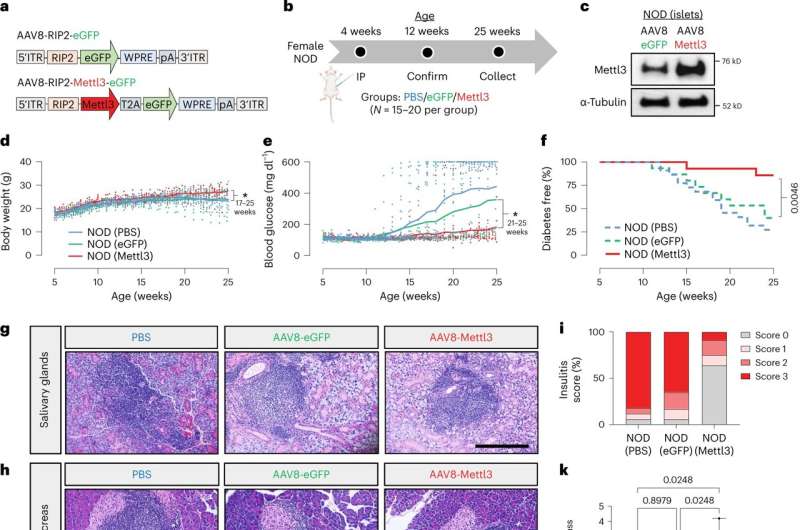
Groundbreaking research by investigators at Joslin Diabetes Center sheds new light on the specific changes β-cells go through at the onset of T1D. Their findings—published in Nature Cell Biology—offer new avenues for targeted interventions for the chronic autoimmune condition.
"In the field of type 1 diabetes, research has largely focused on understanding the immune component, but our study argues that the β-cell is a significant player," said Rohit N. Kulkarni, M.D., Ph.D., Margaret A. Congleton Chair and Co-Head of the Section on Islet & Regenerative Biology at Joslin Diabetes Center.
"Our findings suggest that the β-cell could be initiating key events which then promote the autoimmune mechanism to go awry. It's a paradigm shifting approach."
In a series of experiments with β-cells taken from a mouse model of T1D, as well as from humans with established T1D, Kulkarni and colleagues teased out the complex cascade of biochemical steps called a signaling pathway that controls the innate immune response at the onset of T1D.
The team identified one pathway that influences the immune characteristics of β-cells, acting like control switches that identify them as friend or foe to the body. These control switches can be imagined as tiny tags.
One specific tag the investigators focused on—called N6-methyladenosine (m6A)—plays a vital role in the response of β-cells during T1D onset. By adjusting these control switches, the researchers were able to influence the levels of a crucial protein along this pathway, leading to a notable delay in the progression of the disease in a mouse model of T1D.
Dario F. De Jesus MSc, Ph.D., lead author of the study and Research Associate in the Kulkarni Lab, identified the key enzyme METTL3 as crucial for regulating β-cell antiviral defenses.
In the late stages of T1D, when METTL3 levels were low, it hinted that higher METTL3 levels shield β-cells from dysfunction. By enhancing METTL3 production in the mouse model, the team successfully delayed progression of disease.
"This discovery suggests that interventions to boost METTL3 levels is a potential strategy to protect β-cells and slow down progression of type 1 diabetes," said De Jesus, who is also an Instructor in Medicine at Harvard Medical School.
Taken together, these several lines of evidence paint a clearer picture of the immune events surrounding the still mysterious onset of T1D, including a novel mechanism that could be harnessed for β-cell protection. They also demonstrated that the enzyme METTL3 has the potential to promote β-cell survival and function during disease progression.
"It is notable that this pathway has commercially available compounds that have been used in the context of other diseases," said Kulkarni, who is also a professor of medicine at Harvard Medical School.
"While it's a different target, it's an approach which has been shown to work. Among our next steps, we will focus on identifying specific molecules and pathways that can be harnessed to enhance protection of the β-cell."
More information: Redox regulation of m6A methyltransferase METTL3 in β-cells controls the innate immune response in type 1 diabetes, Nature Cell Biology (2024). DOI: 10.1038/s41556-024-01368-0 www.nature.com/articles/s41556-024-01368-0