This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Long-term analysis reveals SARS-CoV-2 infection and vaccine-induced antibody responses are long-lasting
A long-term analysis conducted by leading microbiologists at the Icahn School of Medicine at Mount Sinai reveals that antibody responses induced by COVID-19 vaccines are long-lasting. The study results, published online in the journal Immunity challenge the idea that mRNA-based vaccine immunity wanes quickly.
The emergence of SARS-CoV-2, the virus that causes COVID-19, in late 2019 sparked the global pandemic that is now in its fifth year. Vaccines that were developed at record speed have saved millions of lives. However, the emergence of SARS-CoV-2 variants and waning immunity have decreased the effectiveness of the vaccines against symptomatic disease. The common perception now is that mRNA-based vaccine-induced immunity wanes quickly.
However, this assumption is largely based on data from short-term studies that include a very limited number of data points following peak responses.
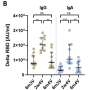
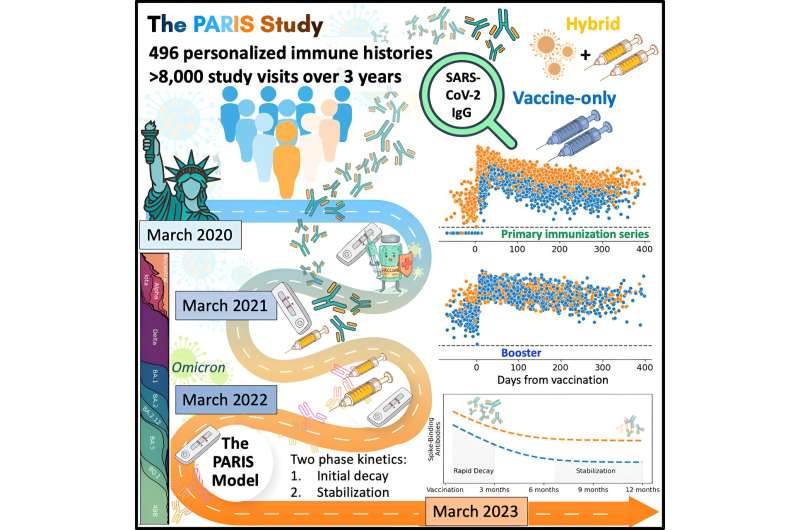
The Mount Sinai research team's analysis of more than 8,000 samples collected over a three-year period in New York City examined how antibody responses to the virus's spike protein changed after infections, during the primary immunization series, during monovalent and bivalent booster vaccination, and during breakthrough infections.
They found that upon primary immunization, participants with pre-existing immunity (those who had previously been infected with the virus) mounted higher antibody responses faster and achieved higher steady-state antibody titers than individuals who had not been previously infected.
The waning of antibody response was characterized by two phases: an initial rapid decay from the strong peak after vaccination, followed by a stabilization phase with very slow decay, suggesting that antibody levels were very long-lasting. Booster vaccination equalized the differences in antibody concentration between participants with and without pre-existing immunity.
Breakthrough infections increased antibodies to similar levels as an additional vaccine dose in individuals who had not previously been infected.
This investigation represents one of the most extensive and in-depth assessments of the longevity of SARS-CoV-2 immune responses to date. Its major conclusion is that changes in the virus that allow it to evade immunity, rather than waning immunity, are the major reason for breakthrough infections.
"Ours is one of the longest-running COVID-19 studies out there," said Viviana Simon, MD, Ph.D., Professor of Microbiology, Medicine and Pathology, Molecular and Cell-Based Medicine, at Icahn Mount Sinai and lead author of the paper.
"Following the same group of people monthly over time is rare and powerful because you can compare immune responses on an individual level. SARS-CoV-2 continues to evolve, so this research is important to provide an understanding of the impact of new variants and new vaccine doses on a healthy immune system and to guide all of us to make the best choices to maintain protection against the virus that continues to circulate in our communities."
This in-depth analysis was made possible through the Protection Associated with Rapid Immunity to SARS-CoV-2 (PARIS) study, an observational, longitudinal cohort of health care workers of the Mount Sinai Health System that was initiated in April 2020. At that time, the densely populated New York metropolitan area was hit with an exponential increase in severe SARS-CoV-2 infections, and essential workers in the health care system were at high risk for infection.
In response to the crisis, a team of leading virologists, physician-scientists, and pathologists at Mount Sinai established a specific and sensitive SARS-CoV-2 binding enzyme-linked immunosorbent assay to measure the SARS-CoV-2 antibody titers accurately. This test was used to measure immune responses in the PARIS cohort in order to determine how quickly the antibody defenses were mounted and how these changed over the months and years of follow-up.
In addition to showing the impact on a person's individual antibody response to vaccines based on the type of vaccine received and whether or not they were infected before receiving the first dose, the PARIS study made possible the development of a mathematical model that can be used to predict and characterize antibody responses of both individual people and populations.
"People have pandemic fatigue, and vaccine uptake has slowed, especially after the vaccines started to be charged to insurance," said Komal Srivastava, MS, Director of Strategy and Operation of the Mount Sinai Center for Vaccine Research and Pandemic Preparedness and co-first author of the paper.
"We were pleasantly surprised to see that the booster doses promoted a large antibody response regardless of a person's personal infection history, so we are hopeful that our study findings will encourage people to get their vaccine boosters when eligible and to stay engaged in research. Our work also showcases the impact of viral evolution over time and why it's critical to keep studies like this going despite the pandemic fatigue."
According to the research team, the PARIS model has broad applications for studying the kinetics of antibodies produced to different COVID-19 vaccines in diverse populations. They stress much more work remains to analyze side effects, applications of the antibody model and continued research about new vaccines and viral variants.
"This study adds an essential piece of data to understand the intricate immune response elicited by SARS-CoV-2 infection and COVID-19 vaccination," says Juan Manuel Carreno Quiroz, Ph.D., Assistant Professor in the Department of Microbiology and co-first author of the paper.
"In light of the emerging viral variants, which predominantly induce a cross-reactive antibody response against the spike protein, it will be exciting to characterize in depth the role of these antibodies—in particular the non-neutralizing ones—in protection against the most recent circulating viral variants."
"Likewise, monitoring the induction of variant-specific antibodies after multiple exposures by breakthrough infections and by administration of updated COVID-19 vaccines, such as the XBB.1.5 monovalent booster, will be key to understanding the evolution of the antibody response over time."
More information: Komal Srivastava et al, SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase, Immunity (2024). DOI: 10.1016/j.immuni.2024.01.017