This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Acetylation: A timekeeper of glucocorticoid sensitivity
Cortisol, also commonly known as the stress hormone, belongs to the family of glucocorticoids. In addition to its physiological function, synthetic derivatives of cortisol are also used as anti-inflammatory drugs. In the cell, the hormone acts by binding to the glucocorticoid receptor (GR), which then acts as a transcription factor to regulate the activity of certain target genes.
In order to switch off the cortisol signal again, the cell breaks down the cortisol-bound GR over time. Therefore, chronic stress or long-term hormone therapy can deplete the cellular GR supply and lead to a loss of sensitivity to cortisol. The molecular mechanisms that lead to this cortisol resistance are still largely unknown.
Understanding the regulatory mechanism of cortisol could enhance the effectiveness of anti-inflammatory therapies and offer strategies to counteract the negative effects of stress- and age-related cortisol excess.
The research group led by Prof. Thorsten Heinzel at the Friedrich Schiller University Jena, together with researchers from the Leibniz Institute on Aging—Fritz Lipmann Institute (FLI) in Jena and the University of Ulm, have now been able to clarify an important aspect of cortisol resistance. The study was recently published in the journal iScience.
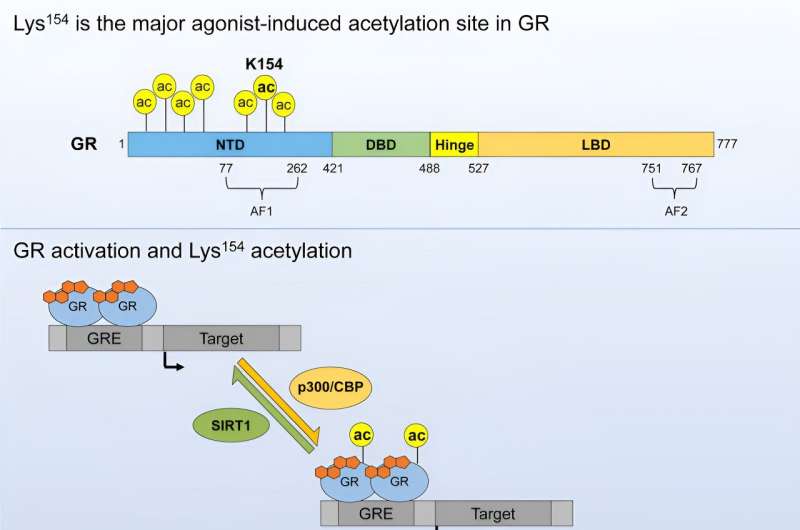
The Proteomics Technology Unit of the FLI was also crucial to the project, providing support with mass spectrometry to investigate the exact molecular composition of the GR. This revealed a chemical modification, known as acetylation, which only occurs when the GR is bound to cortisol. However, this acetylation does not change the function of GR as a transcription factor, as observed from the measurement of gene activity by RNA sequencing, which was also carried out at the FLI by the Next Generation sequencing Core facility.

However, further investigations showed that the modified GR is less stable and is degraded more quickly. "This finding suggests that the cell can adjust its response to cortisol by acetylating GR. Simply put, higher levels of acetylation, shorten the effect of cortisol," explains Dr. Aishwarya Iyer-Bierhoff, the first author of the study.
Elucidation of this regulatory mechanism opens up new possibilities for counteracting cortisol resistance in order to improve the efficacy of anti-inflammatory therapies. In addition, new strategies can be developed to counteract the negative effects of stress- and age-related excess cortisol.
More information: Aishwarya Iyer-Bierhoff et al, Acetylation-induced proteasomal degradation of the activated glucocorticoid receptor limits hormonal signaling, iScience (2024). DOI: 10.1016/j.isci.2024.108943