This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
written by researcher(s)
proofread
Immune cells can adapt to invading pathogens, deciding whether to fight now or prepare for the next battle
How does your immune system decide between fighting invading pathogens now or preparing to fight them in the future? Turns out, it can change its mind.
Every person has 10 million to 100 million unique T cells that have a critical job in the immune system: patrolling the body for invading pathogens or cancerous cells to eliminate. Each of these T cells has a unique receptor that allows it to recognize foreign proteins on the surface of infected or cancerous cells. When the right T cell encounters the right protein, it rapidly forms many copies of itself to destroy the offending pathogen.
Importantly, this process of proliferation gives rise to both short-lived effector T cells that shut down the immediate pathogen attack and long-lived memory T cells that provide protection against future attacks. But how do T cells decide whether to form cells that kill pathogens now or protect against future infections?
We are a team of bioengineers studying how immune cells mature. In our research recently published in the journal Immunity, we found that having multiple pathways to decide whether to kill pathogens now or prepare for future invaders boosts the immune system's ability to effectively respond to different types of challenges.
Fight or remember?
To understand when and how T cells decide to become effector cells that kill pathogens or memory cells that prepare for future infections, we took movies of T cells dividing in response to a stimulus mimicking an encounter with a pathogen.
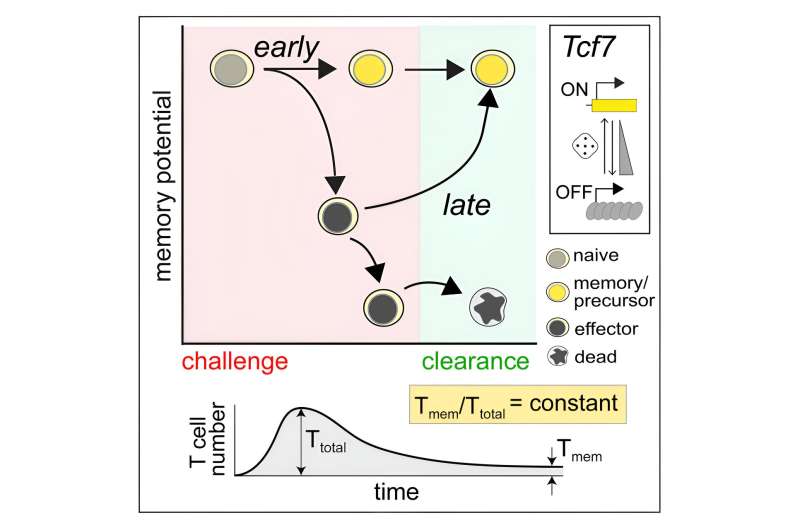
Specifically, we tracked the activity of a gene called T cell factor 1, or TCF1. This gene is essential for the longevity of memory cells. We found that stochastic, or probabilistic, silencing of the TCF1 gene when cells confront invading pathogens and inflammation drives an early decision between whether T cells become effector or memory cells. Exposure to higher levels of pathogens or inflammation increases the probability of forming effector cells.
Surprisingly, though, we found that some effector cells that had turned off TCF1 early on were able to turn it back on after clearing the pathogen, later becoming memory cells.
Through mathematical modeling, we determined that this flexibility in decision making among memory T cells is critical to generating the right number of cells that respond immediately and cells that prepare for the future, appropriate to the severity of the infection.
Understanding immune memory
The proper formation of persistent, long-lived T cell memory is critical to a person's ability to fend off diseases ranging from the common cold to COVID-19 to cancer.
From a social and cognitive science perspective, flexibility allows people to adapt and respond optimally to uncertain and dynamic environments. Similarly, for immune cells responding to a pathogen, flexibility in decision making around whether to become memory cells may enable greater responsiveness to an evolving immune challenge.
Memory cells can be subclassified into different types with distinct features and roles in protective immunity. It's possible that the pathway where memory cells diverge from effector cells early on and the pathway where memory cells form from effector cells later on give rise to particular subtypes of memory cells.
Our study focuses on T cell memory in the context of acute infections the immune system can successfully clear in days, such as cold, the flu or food poisoning. In contrast, chronic conditions such as HIV and cancer require persistent immune responses; long-lived, memory-like cells are critical for this persistence. Our team is investigating whether flexible memory decision making also applies to chronic conditions and whether we can leverage that flexibility to improve cancer immunotherapy.
Resolving uncertainty surrounding how and when memory cells form could help improve vaccine design and therapies that boost the immune system's ability to provide long-term protection against diverse infectious diseases.
More information: Kathleen Abadie et al, Reversible, tunable epigenetic silencing of TCF1 generates flexibility in the T cell memory decision, Immunity (2024). DOI: 10.1016/j.immuni.2023.12.006
This article is republished from The Conversation under a Creative Commons license. Read the original article.![]()