This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Assessing the impact of fast-track drug approval programs
Over the past decade, regulatory agencies such as the Federal Drug Administration (FDA) in the United States and the and the European Medicines Agency (EMA) in the European Union have put programs in place to get new drugs into the hands of patients who need them. The FDA launched its Accelerated Approval (AA) program in 1992, and the EMA started the Conditional Marketing Authorization (CMA) in 2006. These programs allow faster access to new treatments for serious conditions with limited options, including cancer.
But do the programs work? As many advocates and politicians push for even faster drug approvals, some scientists and experts in drug development have pushed back. They cite that the accelerated approvals have not resulted in improved outcomes for patients, and that these programs have even approved drugs that later needed to be withdrawn for safety and efficacy concerns.
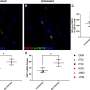
From 2011 to 2022, the FDA granted accelerated approval to 43 indications for cancer treatments. Sixteen of those were later withdrawn.
In a new commentary in Nature Cancer, researchers from the HICCC have reviewed these FDA accelerated approvals that were withdrawn and tracked their outcomes in the EMA. The withdrawals were reviewed and assessed for potential reasons for their failures, including failure to confirm approval data, failure against the standard of care, poor trial design, and changes in the risk-versus-benefit equation.
The researchers found that the causes for withdrawals were nuanced. Apart from the three PI3K inhibitors that were withdrawn because of toxicity and changes to the risk-benefit equation, they found that "it is difficult to argue that harm has resulted" from the fast-track programs.
"There cannot be a perfect system," says Susan Bates, MD, senior author on the study and a physician-scientist at Columbia University Irving Medical Center who focuses on drug development. "As the adage goes, failure is often more interesting than success. We can learn from drug approval failures, increasing our understanding of cancer biology and pharmacology, and ultimately leading to better processes and better therapies for patients."
Both the FDA and the EMA expect some of the approvals will be withdrawn—that is why the withdrawal process exists, the authors maintain. It is in managing that risk with the benefit to patients that the regulatory agencies are walking a tightrope. With their review, the authors argue that overall, these fast-track programs have positively contributed to drug development, bringing potentially lifesaving cancer therapies to those that need them.
More information: George S. Mellgard et al, Lessons from withdrawn accelerated approvals in oncology, Nature Cancer (2024). DOI: 10.1038/s43018-023-00696-8