This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research links age-related inflammation, microglia and Alzheimer's disease
Stanford researchers have uncovered a potential role for a protein called TREM1 in the development of age-related inflammation, cognitive decline, and Alzheimer's disease. This discovery could pave the way for new therapeutic strategies to combat these conditions.
Alzheimer's disease, the most common form of dementia, is characterized by progressive memory loss and cognitive decline. Aging is the primary risk factor for Alzheimer's, and both are associated with chronic inflammation (sometimes dubbed "inflamm-aging") and impaired immune responses. Recent genetic studies have suggested that a class of immune cells called myeloid cells, which are found both in the brain and throughout the body, may play a key role in the development of Alzheimer's.
In a study published in the journal Nature Neuroscience Stanford neuro-immunologist Katrin Andreasson and a wide-ranging team delved into the role of TREM1—a protein known to intensify inflammatory responses in myeloid cells—in Alzheimer's disease and the aging brain.
While its counterpart, TREM2, had been linked to Alzheimer's, TREM1's role in the disorder had remained a mystery. Through the use of mouse models and analysis of postmortem human brain tissue, the wide-ranging research team—spearheaded by senior scientist Edward N. Wilson—aimed to shed light on TREM1's influence on Alzheimer's and cognitive aging.
TREM1 linked to inflammation, memory loss, and Alzheimer's pathology
First, the research team looked at aging mice with and without normal levels of TREM1. Normally, this protein is highly expressed in myeloid cells of the peripheral immune system (i.e. not in the brain).
Surprisingly, removing this protein seemed to make peripheral myeloid cells more resilient to the effects of aging: resisting age-related changes in cellular energy metabolism and inflammation. This also had beneficial effects on the brain, reducing age-related memory loss and mitigating bioenergetic changes typically induced by amyloid-β42, a protein that forms plaques in the brains of Alzheimer's patients.
The researchers then studied the effects of reducing the levels of TREM1 in an established mouse model of Alzheimer's Disease. They found that lowering TREM1 prevented memory loss in these animals, preserved the normal structure of microglial cells (a type of myeloid cell found in the brain), and reduced changes in the brain associated with the disease.
"There is a broad consensus that microglia, the resident macrophages of the brain, are critical in maintaining brain health, for example by clearing proteins like amyloid that accumulate in Alzheimer's disease," said Andreasson, who is the Edward F. and Irene Thiele Pimley Professor of Neurology at Stanford and a Wu Tsai Neurosciences Institute affiliate.
"What was surprising for us was that peripheral macrophages—immune cells that reside outside of the brain—actually had a larger role in maintaining healthy cognitive function both in aging and models of AD."
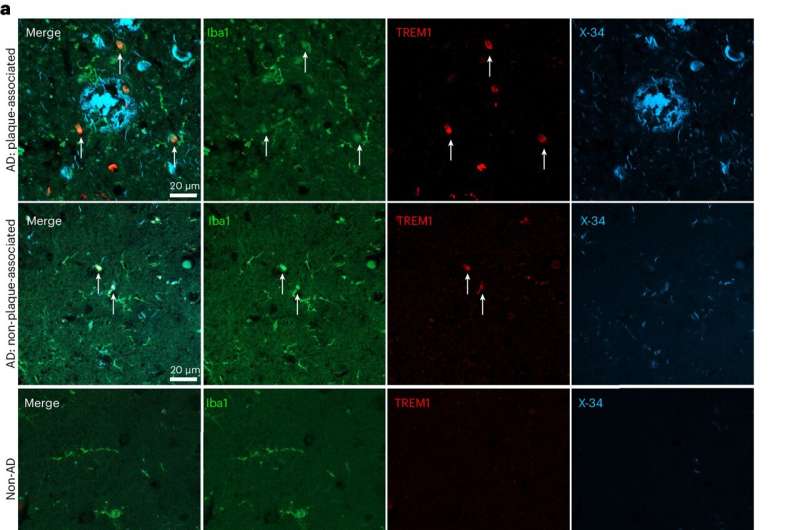
Finally, the researchers examined postmortem human brain tissue from Alzheimer's patients. The team observed heightened TREM1 levels in myeloid cells surrounding amyloid plaques and noted that higher TREM1 levels in the brain appeared to correlate with increased disease severity.
"This study introduces two new concepts," Andreasson said.
"First, peripheral macrophages, where TREM1 appears to be highly expressed, appear to have a large influence on brain function and in fact may influence the function of microglia. Second, with aging, immune cells become very energy-depleted and unable to maintain healthy immune responses; we found that reducing levels of TREM1 prevented this metabolic decline and restored immune responses that were supportive of healthy brain function."
Research could provide new leverage to reduce 'inflammaging' and slow neurodegeneration
The study highlights the intricate interplay between our immune systems and brain health, the authors say, suggesting that cognitive decline in aging and Alzheimer's disease may be influenced by metabolic and functional dysfunction of peripheral myeloid cells.
Their findings suggest that restoring peripheral immune cells to a more resilient, youthful state—for example by tuning down their TREM1 levels—could reduce both inflammation and cognitive decline.
With the support of an Innovation Award from the Knight Initiative, the authors are eager to pursue this approach as a promising strategy for slowing or even halting the progression of Alzheimer's disease and potentially other age- and inflammation-related brain disorders. However, further research will be needed to fully understand the mechanisms involved and to develop effective therapies based on these findings.
"A very interesting question is how the detrimental effect of TREM1 in peripheral macrophages gets transmitted to the brain," Andreasson said. "We are actively investigating this complex question in the lab, and hope to have some answers soon."
More information: Edward N. Wilson et al, TREM1 disrupts myeloid bioenergetics and cognitive function in aging and Alzheimer disease mouse models, Nature Neuroscience (2024). DOI: 10.1038/s41593-024-01610-w