This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New human cell-based 3D model reveals insights into how immune cells contribute to Alzheimer's disease
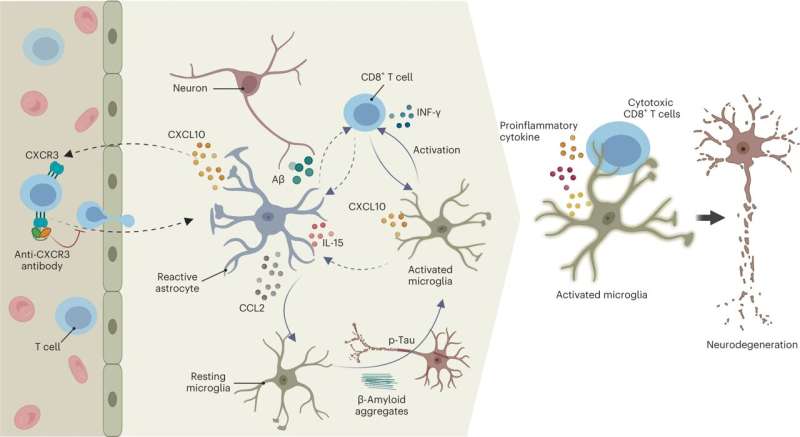
Cognitive decline associated with Alzheimer's disease (AD) develops when neurons begin to die, which can be caused by inappropriate immune responses and excessive inflammation in the brain triggered by amyloid beta deposits and tau tangles, two hallmarks of the disease.
Also, immune cells outside of the brain, particularly T cells, can enter the brain and worsen AD pathology, but studying this process has been difficult.
Now, a team led by researchers at Massachusetts General Hospital (MGH) has engineered a novel 3D human cellular model that mimics the intricate interactions between brain cells and these immune invaders.
The work, which builds on previous 3D models of AD developed by the team, is described in Nature Neuroscience.
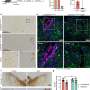
In the new study, the team used the model to demonstrate that as Alzheimer's pathology accumulates in the brain, specific types of immune cells called CT8+ T Cells surge into the brain and amplify the destruction caused by neuroinflammation.
The team also identified the molecular mechanisms that drives the infiltration of T cells to the brain and showed that blocking these mechanisms reduced the destructive effects of T cell infiltration.
The findings could lead to new therapies for Alzheimer's patients that target immune cell infiltration in the brain.
"Enabled by cutting-edge microfluidic technology, this model opens up a window to observe infiltrating peripheral immune cells in action within 3D cell cultures; their interactions with brain cells; and their impact on neuroinflammation and neurodegeneration," says co-lead author Mehdi Jorfi, Ph.D., instructor in Neurology at MGH.
"We hope our work contributes to developing a more physiologically relevant human Alzheimer's disease model in a dish," adds co–senior author Doo Yeon Kim, Ph.D., associate professor of Neurology at MGH.
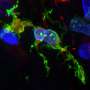
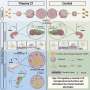
The team's new model is a 3D human neuroimmune axis model is comprised of stem-cell derived neurons, astrocytes and microglia along with peripheral immune cells.
The model is an extension of previous work done by the research team to create and validate 3D lab models of AD that better replicate the hallmark plaques and tangles of the disease in a three-dimensional environment—much in the same way the disease develops in the brain.
In addition to observing higher levels of T cells in AD brain models, the team identified a pathway between a chemokine (CXCL10) and chemokine receptor (CXCR3) that plays a key role in regulating T cell infiltration.
Blocking this pathway largely prevented T cell infiltration and neurodegeneration in AD cultures.
The findings could help in identifying new therapeutic targets that slow or halt the infiltration of T cells into the brains of Alzheimer's patients, and potentially reduce the devastating cognitive impacts of the disease.
"This multidisciplinary research approach identified the different behaviors of distinct cell types in this disease context, and we aim to shed light on the underlying mechanisms to identify strategies for intervention that could lead to more effective treatments," said co–lead author Joseph Park, Ph.D., instructor in Neurology at MGH.
Additional targets may be identified with continued experiments with this model.
"Perhaps, what is most exciting about this study is that we have identified a new drug target on T cells, outside of the brain, which would be more accessible to novel treatments, especially since it has been traditionally difficult to get drugs into the brain," says senior author Rudolph Tanzi, Ph.D., director of the Genetics and Aging Research Unit at MGH.
More information: Mehdi Jorfi et al, Infiltrating CD8+ T cells exacerbate Alzheimer's disease pathology in a 3D human neuroimmune axis model, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01415-3
The code for analysis and generating scRNA-seq figures can be found at github.com/jospark09/PiChip and zenodo.org/record/8150286.