This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Medical devices review calls for immediate action on unfair biases to prevent patient harm
A report published Monday, 11 March 2024, details the findings of the Independent Review of Equity in Medical Devices.
It calls for concerted action on devices that are prone to unfair biases, including pulse oximeters and those enabled by Artificial Intelligence (AI), to prevent patient harm.
Set up in 2022 by the then Secretary of State for Health and Social Care, the review sought to establish the extent and impact of ethnic and other unfair biases in the performance of medical devices commonly used in the NHS. It was commissioned amid concerns that such biases may lead to suboptimal treatment for the affected groups in the population.
The review focused on three types of medical device where evidence suggested that the potential for harm was substantial. These were optical devices such as pulse oximeters, AI-enabled devices and certain genomics applications, such as polygenic risk scores.
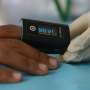
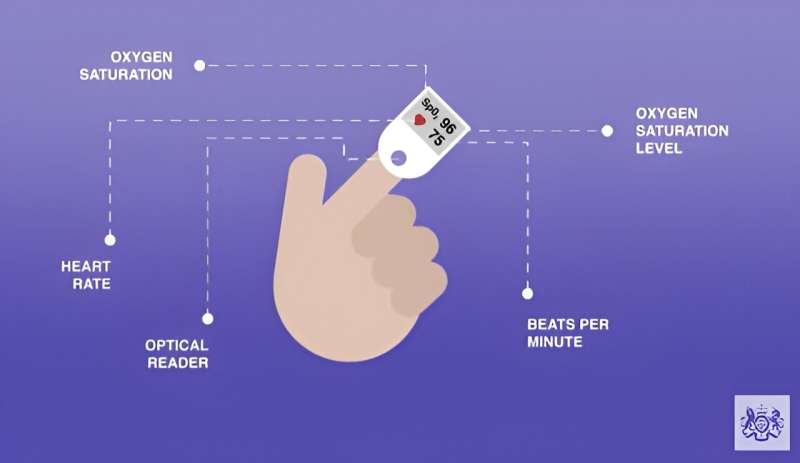
The expert panel found evidence that pulse oximeters—widely used during the COVID-19 pandemic to monitor blood oxygen levels—can over-estimate the amount of oxygen in the blood of people with darker skin tones. This could lead to delay in treatment if dangerously low oxygen levels were missed.
The review recommends mitigating actions in relation to pulse oximeters already in widespread use across the NHS. Further recommendations aim to prevent adverse impacts arising in new devices as they are developed and put into use.
On AI-enabled devices, the review found evidence of potential biases against women, ethnic minority and disadvantaged socioeconomic groups in how clinical decision-making tools select higher risk patients needing more intensive treatment.
One example is the potential under-diagnosis of skin cancers for people with darker skin when using AI-enabled devices. This is as a result of machines being "trained" predominantly on images of lighter skin tones. There is also a long-standing problem of under-diagnosis of cardiac condition in women, which AI algorithms in medical devices could make worse.
The University of Liverpool's Professor Dame Margaret Whitehead, Chair of the Review, said, "The advance of AI in medical devices could bring great benefits, but it could also bring harm through inherent bias against certain groups in the population, notably women, people from ethnic minorities and disadvantaged socio-economic groups.
"Our review reveals how existing biases and injustices in society can unwittingly be incorporated at every stage of the lifecycle of AI-enabled medical devices, and then magnified in algorithm development and machine learning.
"Our recommendations therefore call for system-wide action by many stakeholders and now need to be implemented as matter of priority with full government support."
The review also recommends that the Government should start preparing now for the disruption to health care from the next generation of AI-enabled machines if it is to minimize the risk of patient harm.
Panel member Professor Chris Holmes warned that the Government needs to understand how the advent of large language and foundation models like ChatGPT will disrupt clinical and public health practices.
"We are calling on the government to appoint an expert panel including clinical, technology and health care leaders, patient and public representatives and industry to assess the potential unintended consequences arising from the AI revolution in health care.
"Now is the time to seize the opportunity to incorporate action on equity in medical devices into the overarching global strategies on AI safety," he said.
More information: Independent Review of Equity in Medical Devices report. www.liverpool.ac.uk/research/r … -in-medical-devices/