This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Micro-patterning: A new system to induce alveolar and airway epithelial cells
Professor Shimpei Gotoh and Junior Associate Professor Kazuo Takayama teamed up in study to construct a novel in vitro culture system for alveolar and airway epithelial cells, employing a biomaterials engineering method known as micro-patterning technique, and using the newly devised system to simulate viral infections by SARS-CoV-2 variants. The study is published in the journal Stem Cell Reports.
The respiratory epithelium lining the airway and alveoli, where oxygen and carbon dioxide exchange occurs, consists primarily of two cell types, type 1 and 2 alveolar epithelial cells, referred to as AT1 and AT2, respectively. While AT1 cells are thin and flat, optimal for mediating gas exchange, cuboidal AT2 cells secrete surfactant proteins and act as tissue stem cells.
With their abilities of self-renewal and AT1 differentiation, AT2 cells are of particular interest in regenerative medicine to facilitate the repair of injured or diseased lungs. The Gotoh Laboratory has previously devised a protocol to use iPS cells as a valuable starting material to generate AT2 cells.
However, the requirement for Matrigel-based 3D culture limits culture size and direct image-based analysis. Furthermore, cells in such systems typically orient themselves with their apical surface facing inward, thus unable to model accurately in vivo tissue structure for mechanistic studies.
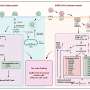
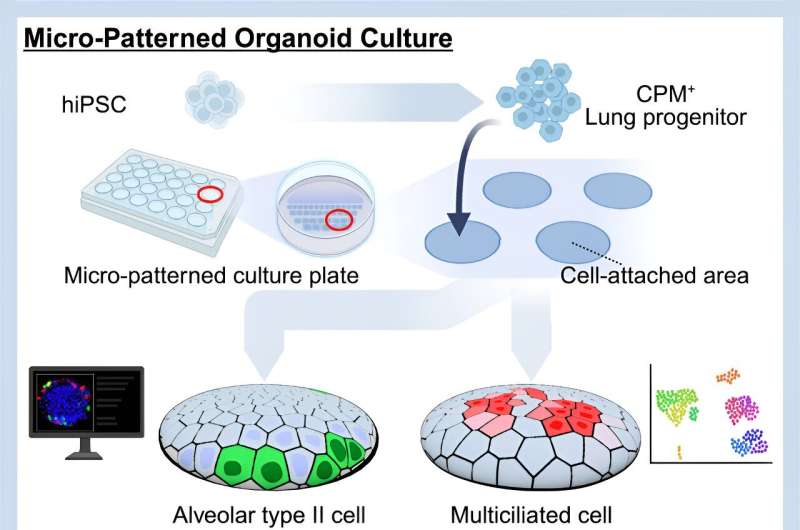
In an attempt to create a better means to differentiate and grow iPS cell-derived AT2 cells, non-adhesive 2.5-dimensional (D) cell culture plates were micro-patterned with tiny circles of cell-adhesive coating, 100 or 200 μm in diameter (for comparison, the diameter of human hair ranges from 17-181 μm).
First, to ensure that such micro-patterned plates are compatible with AT2 cell induction, iPS cell-derived lung progenitor cells that express a green fluorescent protein upon differentiation into AT2 cells were seeded and treated with a culture medium specifically for alveolar epithelial cell induction.
Compared to conventional 2D monolayer cultures, the relative proportion of fluorescent cells and the expression of SFTPC, a gene encoding one of several surfactant proteins, were significantly higher in the micro-patterned system than in conventional cultures. Notably, NaPi2b protein was observed on the edge of colonies formed on the micro-patterned cell-adhesive coating, indicative of an "apical-out" orientation for these AT2 cells.
During this study, the researchers observed that a DNA-binding fluorescent dye (Hoechst-33342) stained peripheral live cells much more rapidly than those in the center. By separating cells based on their fluorescence intensity, they found more cells with green fluorescence and AT2 marker genes to be enriched in the edge (Hoechst-33342high) subpopulation compared to the center (Hoechst-33342low) subpopulation, further indicating AT2 cells to be on the periphery.
The research team next separated edge and center cells and analyzed their gene expression profiles by bulk RNA sequencing (RNA-seq). Among nearly 2,000 differentially expressed genes, several AT2 marker genes and endogenous WNT suppressor genes were significantly upregulated in the peripheral subpopulation compared to undifferentiated lung progenitor cells, indicative of suppressed WNT signaling in AT2 cells at the periphery. In contrast, the gene expression profile suggested Notch signaling activation in the core subpopulation.
Next, to demonstrate that this micro-patterned system can generate multiciliated airway epithelial cells, lung progenitor cells were treated with a medium to promote differentiation towards mature airway epithelium. As a result, gene expression, immunofluorescence, and electron microscopic analyses detected the upregulation of multiciliated cell markers genes (FOXJ1 and SNTN) and proteins (FOXJ1 and acetylated tubulin), as well as moving cilia in multiciliated cells.
Moreover, the micro-patterned system was more efficient at generating FOXJ1+ cells than conventional 2D cultures. By bulk RNA-seq, the researchers discerned that genes related to goblet and mucous cell lineages were similarly upregulated in both the edge and center subpopulations compared to lung progenitor cells, with the center subpopulation also showing upregulation of several marker genes associated with multiciliated cells, in addition to an enrichment of multiple gene ontology (GO) terms with its differentially upregulated genes.
The researchers also analyzed the various cell types in the micro-patterned colonies by single-cell (sc)RNA-seq induced into both epithelial cell types. Alveolar epithelial cells were primarily segregated into three clusters: AT2 cells characterized by high expression of AT2 marker genes, proliferating AT2 cells with low marker expression, and respiratory bronchiole-like cells.
Conversely, a multiciliated cluster expressing SNTN and FOXJ1 and multiple clusters representing relatively immature ciliated epithelial cells (FOXJ1, DRC1, and CCNO) or immature secretory cells (SERPINA1 and MUC5B) were identified in the airway epithelial cells. Interestingly, an independent cluster formed by cells in the periphery displayed transcriptomic changes resembling cells previously described as intermediate cells on the differentiation trajectory from AT2 to AT1 cells.
The research team next infected the micro-patterned alveolar or airway epithelial cultures with five SARS-CoV-2 variants, B.1.1.214, B.1.617.2 (delta), and omicron variants BA.1, BA.2, and BA.5, to examine the feasibility of using this new system to study infections by respiratory viruses.
Notably, whereas alveolar epithelial cells infected with the B.1.1.214 and B.1.617.2 variants readily released viruses, infections by the omicron variants led to much lower virus release. B.1.1.214-, B.1.617.2-, and BA.5-infected airway epithelial cells followed a similar trend, similarly releasing high amounts of virus three days post-infection (dpi), but significantly declined on four dpi after infection by B.1.617.2 or BA.5. In contrast, airway epithelial cells infected by BA.1 and BA.2 released negligible levels of virus.
Similarly, when viral gene expression was examined, B.1.617.2 displayed high viral tropism, as both epithelial cell types showed dramatic increases in viral gene expression and protein levels. Notably, B.1.1.214 and BA.1 showed weak and strong specificity for alveolar and airway epithelial cells, respectively, as viral gene expression and protein levels in the specified epithelial cells were higher than the other.
Lastly, the research team examined gene expression profiles of alveolar and airway epithelial cells due to infection by B.1.617.2 and BA.1. While B.1.617.2-infected alveolar and airway epithelial cells and BA.1-infected airway epithelial cells all showed an interferon response, airway epithelial cells infected by B.1.617.2 underwent gene expression changes indicative of apoptosis (programmed cell death), which was confirmed by independent assays.
Using this micro-patterned culture system, the research team successfully created a means to culture AT2 cells in the correct orientation (with their apical surface facing the medium) that is amendable to imaging.
The researchers also demonstrated the applicability of alveolar and airway epithelial cells induced in the micro-patterned system to reveal variant-specific viral features like viral tropism, a property that makes the system adaptable for disease modeling and drug discovery.
More information: Atsushi Masui et al, Micro-patterned culture of iPSC-derived alveolar and airway cells distinguishes SARS-CoV-2 variants, Stem Cell Reports (2024). DOI: 10.1016/j.stemcr.2024.02.011