This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Study: PR55α-controlled PP2A inhibits p16 expression and blocks cellular senescence induction
A new research paper was published in Aging titled, "PR55α-controlled protein phosphatase 2A inhibits p16 expression and blocks cellular senescence induction by γ-irradiation."
Cellular senescence is a permanent cell cycle arrest that both internal and external genotoxic stressors, such as telomere dysfunction and DNA damage can trigger. The execution of senescence is mainly by two pathways, p16/RB and p53/p21, which lead to CDK4/6 inhibition and RB activation to block cell cycle progression. While the regulation of p53/p21 signaling in response to DNA damage and other insults is well-defined, the regulation of the p16/RB pathway in response to various stressors remains poorly understood.
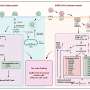
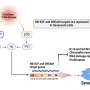
In this new study, researchers Chitra Palanivel, Lepakshe S. V. Madduri, Ashley L. Hein, Christopher B. Jenkins, Brendan T. Graff, Alison L. Camero, Sumin Zhou, Charles A. Enke, Michel M. Ouellette, and Ying Yan from the University of Nebraska Medical Center report a novel function of PR55α, a regulatory subunit of PP2A Ser/Thr phosphatase, as a potent inhibitor of p16 expression and senescence induction by ionizing radiation (IR), such as γ-rays.
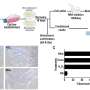
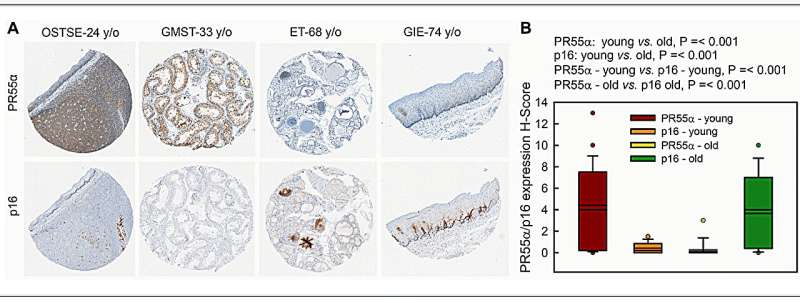
"During natural aging, there is a gradual accumulation of p16-expressing senescent cells in tissues. To investigate the significance of PR55α in this up-regulation of p16, we compared levels of the p16 and PR55α proteins in a panel of normal tissue specimens derived from young (≤43 y/o) and old (≥68 y/o) donors," write the researchers.
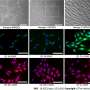
The results show that ectopic PR55α expression in normal pancreatic cells inhibits p16 transcription, increases RB phosphorylation, and blocks IR-induced senescence. Conversely, PR55α-knockdown by shRNA in pancreatic cancer cells elevates p16 transcription, reduces RB phosphorylation, and triggers senescence induction after IR.
Furthermore, this PR55α function in the regulation of p16 and senescence is p53-independent because it was unaffected by the mutational status of p53. Moreover, PR55α only affects p16 expression but not p14 (ARF) expression, which is also transcribed from the same CDKN2A locus but from an alternative promoter. In normal human tissues, levels of p16 and PR55α proteins were inversely correlated and mutually exclusive.
"Collectively, these results describe a novel function of PR55α/PP2A in blocking p16/RB signaling and IR-induced cellular senescence," the authors conclude.
More information: Chitra Palanivel et al, PR55α-controlled protein phosphatase 2A inhibits p16 expression and blocks cellular senescence induction by γ-irradiation, Aging (2024). DOI: 10.18632/aging.205619