This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Versatile antibody technology allows design of long-acting antibodies with tailored target-dependent mode of actions
Antibody therapeutics are a rapidly growing class of drugs used to treat infections as well as a range of diseases. Among them are cancer and autoimmunity particularly important, and the use of antibody therapeutics transforms the lives of patients. Thus, there is an intense interest in engineering new antibody formats with improved efficacy for both therapeutic and prophylactic use.
To engineer the next generation of antibodies, one must carefully consider which of the antibody effector functions should be maintained, enhanced, or abolished. To achieve potent and specific treatment with minimal risk of side effects this is crucial. In all cases, the pharmacokinetic profile of the antibody will guide dosing size and frequency, so as to secure high concentration of active antibody in blood and tissue, and with as much as possible reaching the target over time.
In a Nature Communications paper, the laboratory of Professor Jan Terje Andersen and collaborators report on a novel antibody constant region (Fc) variant that is designed to give antibodies broad biodistribution and make them long-acting in the body.
These features allow the antibodies to reach the site of action at high concentrations, and the improvements in pharmacokinetics are expected to increase patient convenience, treatment adherence and reduce costs for the health care system. This is particularly important for treatment of life-long chronic diseases and for prophylactic treatment of severe infections.
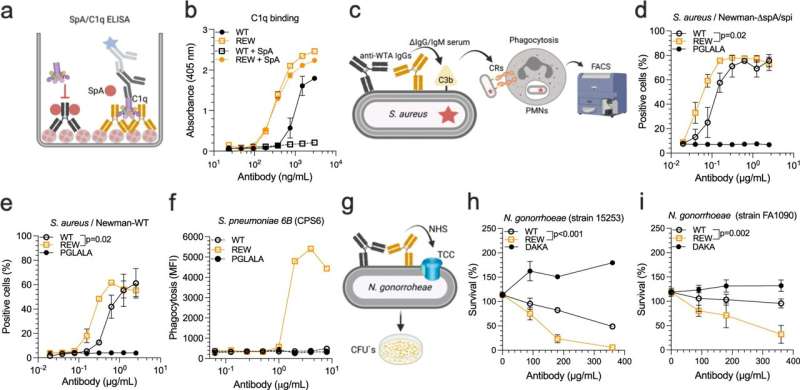
The technology reported is based on three amino acid substitutions (Q311R/M428E/N434W) (REW), which together improve pH-dependent binding of the antibody Fc to human FcRn, a broadly expressed cellular receptor that controls the plasma half-life and biodistribution of IgG antibodies.
The authors demonstrate that antibodies with such an Fc region have prolonged plasma half-life and improved biodistribution in human FcRn-expressing mice, and furthermore, allow for both invasive and non-invasive delivery.
Importantly, the "REW technology" also increases engagement of the complement system and thus provides antibodies with enhanced ability to kill cancer cells and gram-positive as well as gram-negative bacteria. This property depends on which of the four human IgG subclasses that are used as design scaffold. For instance, while REW-containing IgG2 is equipped with ability to induce complement activation, IgG4-REW remains inert in this regard.
This is a differentiator compared to other reported Fc-engineered strategies. Thus, this engineering approach will be a useful tool for design of IgG antibodies with tailored mode-of-action towards a specific target and indication.
"Development of the next generation of tailored antibodies will depend on engineering that secures specific modes of action without causing severe side effects. For instance, antibodies targeting cancer cells, viruses or bacteria will benefit from features that specifically enhance target eradication," says Jan Terje Andersen, the study's senior author.
"In addition, engineering that prolongs the exposure time, systemically, but also at mucosal sites, and allows antibodies to reach the site of action at high concentrations is favorable, as it may extend dosing intervals. The REW technology has several of these features and should therefore be attractive in design of antibodies to fight cancer and to improve management of chronic diseases as well as severe infections.
"In all these cases, the duration of action of a given antibody, or cocktail of antibodies, is key to secure effective long-acting treatment. Such a technology may also make antibodies more affordable for global access."
The project was led by the Laboratory of Homeostasis and Adaptive Immunity, headed by Professor Jan Terje Andersen, which is part of Precision Immunotherapy Alliance (PRIMA), a Norwegian Research Council Center of Excellence, University of Oslo and Oslo University Hospital.
The research was carried out in collaboration with scientists from Norwegian Institute of Public Health, University Medical Center Utrecht, University of Massachusetts Chan Medical School, Christian-Albrechts University Kiel, University of Copenhagen, Roche Pharma Research and Early Development, International AIDS Vaccine Initiative and Translational Health Science & Technology Institute in India.
More information: Stian Foss et al, Human IgG Fc-engineering for enhanced plasma half-life, mucosal distribution and killing of cancer cells and bacteria, Nature Communications (2024). DOI: 10.1038/s41467-024-46321-9