This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Antibody-based therapies may be effective in fight against influenza B
With a new flu season upon us, scientists have been testing new ways of fighting the deadly disease with antibody-based therapies—and they've discovered that this approach may be effective in combating influenza B.
Dr. Hillary Vanderven, a lecturer in immunology and infectious diseases based at James Cook University's Australian Institute of Tropical Health and Medicine, was the lead author of the study published in JCI Insight.
She said antibody-based therapies for respiratory viruses are of increasing importance as they can be a safe and effective tool to treat severe respiratory infections, especially in high-risk groups.
"Antibodies are proteins made by the immune system that can target specific molecules on pathogens," said Dr. Vanderven.
She said antibody-based therapeutics are safe, but clinical trials have generally shown minimal or no impact on influenza A, and there are currently no approved antibody-based therapies to treat human influenza.
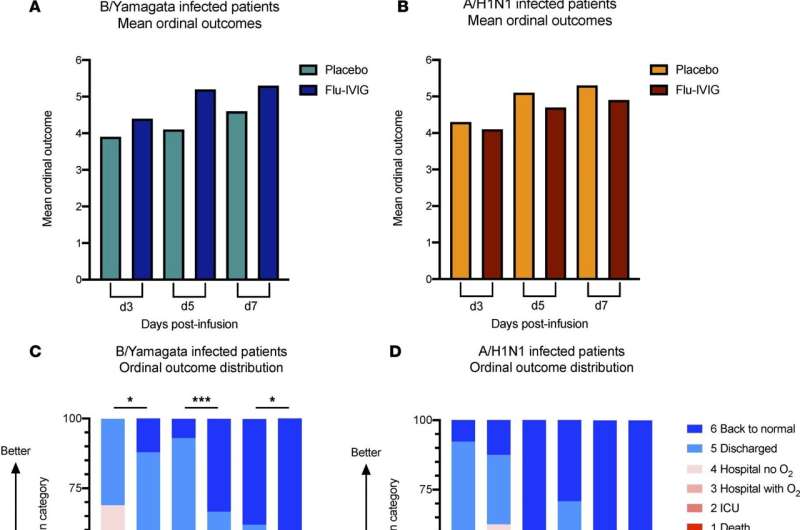
"A recent clinical trial used hyperimmune intravenous immunoglobulin (Flu-IVIG)—purified from donors who were vaccinated against or had recovered from flu—which contained antibodies to fight flu infections. This new therapy was tested on 308 patients hospitalized with severe influenza A or B," said Dr. Vanderven.
Flu-IVIG treatment improved outcomes in patients with influenza B but showed no benefit for influenza A. In this new study, researchers wanted to understand why Flu-IVIG therapy was only effective for influenza B by measuring different types of antibodies.
Dr. Vanderven said their findings suggest that certain types of influenza antibodies, that are capable of killing infected cells, may assist in recovery from severe influenza B but not influenza A.
She said the need for antibody-based treatments targeting respiratory viruses has become increasingly urgent. The demand for an expanded arsenal of anti-viral therapies to combat severe respiratory infections is high, with influenza, COVID-19 and RSV all co-circulating in the community.
"Our comprehensive examination of serum antibodies has provided invaluable insight into the mechanisms and antibody characteristics that underpin effective humoral immunity against influenza virus. This knowledge will help to inform the development of new and improved antibody-based therapies."
More information: Hillary A. Vanderven et al, Understanding the treatment benefit of hyperimmune anti-influenza intravenous immunoglobulin (Flu-IVIG) for severe human influenza, JCI Insight (2023). DOI: 10.1172/jci.insight.167464