This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
reputable news agency
proofread
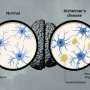
Do you really want to find out if you'll get Alzheimer's?

A few years ago, researchers made the unnerving discovery that in the brains of people with Alzheimer's disease, disordered clumps of abnormal proteins had been growing for 15 or even 20 years before their diagnosis. That means these pathological-looking deposits are silently accumulating in the brains of millions of seemingly healthy individuals in their 50s and 60s.
Recently, scientists have found that a blood test can detect that silent damage with surprising accuracy. About 13% of people ages 75–84 have Alzheimer's disease, which means a substantial fraction of younger people ought to test positive. But are we better off knowing?
There are few Alzheimer's drugs for people with symptoms—and nothing for presymptomatic people. The leading drugs are expensive antibody infusions that clear out most of the visible deposits, called amyloid, but don't slow the degeneration of neurons. These have shown only a modest ability to stall the disease's progression. Nothing can reverse its course.
The blood test that's causing all the excitement measures levels of a protein called p-Tau 217. A study published in Nature Medicine and another published in January in the Journal of the American Medical Association showed that this test works as well as other Alzheimer's diagnostics—PET scans and cerebrospinal fluid sampling following a lumbar puncture. That means it's likely not just a predictor of risk, but an indicator that something is already wrong in your brain.
Some doctors envision Alzheimer's tests becoming as routine as a cholesterol workup—though of course, the results are likely to be far more terrifying and, for now, dramatically less actionable.
The test works so well because "It really reflects the core pathology of Alzheimer's disease," said Henrik Zetterberg, a professor of neurochemistry at the University of Gothenburg in Sweden. The disease can start when a protein called beta amyloid collects outside of neurons, but that alone won't necessarily cause impairment—so tests for beta amyloid are not very predictive.
The progression to true Alzheimer's begins when changes happen within the neurons, including another protein buildup called tau tangles. At that stage, neurons start to shrink back, and in the process, produce a modified protein—p-tau 217.
But it's not clear what people can do with the knowledge that they have elevated p-tau 217. Scott Small, a neurologist at Columbia University, said that the question recently came up in a conversation with his colleagues, and most of them said they'd take one of the available antibody drugs.
Those drugs do an extraordinary job of cleaning out those amyloid plaques. These are the most visible sign of the disease upon autopsy—like "wiry nests" contaminating the brain, Small said. But scientists still don't agree on the connection between the amyloid plaques and the cognitive effects of the disease. The drugs don't clear out the tau tangles or stop neurons from dying, he said. At best, they slow the progression of the disease by about 30%.
The first of these antibody drugs to win approval from the Food and Drug Administration was Biogen's aducanumab, hailed as a blockbuster with an individual price tag of $56,000 a year. But ultimately, Biogen abandoned it due to concerns about inconsistent clinical trials and serious side effects. Last year, the FDA approved a similar drug, lecanemab, which showed more consistent evidence for a modest slowing of symptoms, but also a risk of brain swelling and bleeding.
Some doctors worry that the pharmaceutical industry will take advantage of the fear surrounding Alzheimer's to sell more of these expensive drugs to people unlikely to benefit.
Last year, the Alzheimer's Association, working with a panel scientists, floated a proposal to label cognitively normal people as having "Stage 1 Alzheimer's Disease" if they test positive for blood biomarkers such as p-tau 217. Eric Widera, a professor of medicine and geriatrician at the University of California San Francisco, said many of the panelists had ties to the pharmaceutical industry. Some stand to make money if they can redefine the disease to include asymptomatic people, and the relabeling offered no clear-cut benefit to patients, who might face emotional distress, stigma and discrimination if the information got out.
No test is perfect, so there would inevitably be some false positives or people whose degeneration was so slow they were likely to die of something else before they noticed symptoms. Widera worried that the tests might quietly find their way into standard blood panels, after which millions of people would be horrified to be told they have Alzheimer's.
Despite these concerns, researchers are elated at the power of this blood test for accelerating their progress toward better treatments.
No antibody drugs are approved for asymptomatic people, but there's a gray area since most of us have occasional mental lapses. And doctors may prescribe the drugs off-label. It will take time and good epidemiological studies to establish how to interpret the p-tau 217 test. How high a reading should be considered abnormal or warrant treatment?
It's also possible that the existing antibody drugs will prevent disease if given early enough. Reisa Sperling, a Harvard researcher, is conducting clinical trials in people without symptoms, using the p-tau 217 test to screen volunteers.
Others are taking a different approach—one that they hope gets closer to the root cause of the disease. Small, of Columbia, said he's examining changes in the way proteins are transported within cells. He made an analogy with cholesterol deposits in arteries. You can aim drugs at breaking down the deposits, but it's more effective to use drugs that prevent the liver from making excessive cholesterol in the first place.
In the researchers' wildest hopes, preventive drugs will get so good that a positive test for Alzheimer's proteins would be no more frightening than a high cholesterol reading. They have a long way to go.
2024 Bloomberg L.P. Distributed by Tribune Content Agency, LLC.